Authorised Representative: Full Guide on Regulation (EU) 2019/1020 and How to Avoid Legal Pitfalls When Selling Products to the EU
2 April 2023 Law & Legislation
In this post I’m going to tell you everything you need to know about Regulation (EU) 2019/1020 on Market Surveillance and the need for most non-EU businesses to have an authorised representative located in the EU. Read this guide if you want to avoid legal pitfalls and prevent customs not allowing your products to enter the EU, or marketplaces such as Amazon blocking your products from being sold on the European market.
Let’s dive right in.
Table of Contents
Regulation (EU) 2019/1020 in a nutshell
Economic operators and other definitions
How Regulation (EU) 2019/1020 protects the internal market from unsafe products
Why would I need an Authorised Representative?
What products need an Authorised Representative?
Does Regulation (EU) 2019/1020 apply when I sell products in the UK?
What are potential legal pitfalls when I would not have an economic operator within the EU?
I am a Fulfillment Service Centre: What happens if I don’t appoint an Authorised Representative?
Do I need an Authorised Representative when I sell products in the UK?
How to avoid legal pitfalls and keep selling products on the European market
What are the tasks of an authorised representative?
What do specific directives and regulations say about the AR?
Is an Authorised Representative legally responsible for defective products?
Can I use my distributor as my Authorised Representative?
5 Steps To Choosing a European Authorised Representative
Companies offering Authorised Representative services
Prevent your products from being blocked from entry into the EU
Regulation (EU) 2019/1020 in a nutshell
With Regulation (EU) 2019/1020 coming into force, every business from outside the EU selling products directly or indirectly (through ecommerce platforms) should have either an importer or an authorised representative located in the EU, acting as a liaison between the manufacturer based outside of the EU and a national authority within the EU. The tasks of the economic operator, such as verifying that there is a technical file and declaration of conformity present, are determined in the Regulation.
The address of this economic operator must be clearly visible on the product or packaging.
This regulation also means that Fulfilment Service Providers, such as Amazon, are considered “economic operators”. This means that whenever you sell a product on Amazon, Amazon will become, amongst others, responsible for verifying and maintaining the technical file and declaration of conformity (if they not take other measures and transfer this responsibility to another economic operator).
Watch this webinar about the ins & outs of Regulation (EU) 2019/1020:
Economic operators and other definitions
Before we proceed, let's start with some definitions first, so we’re clear on what these terms mean.
Here are some of the most important terms you will see come up, and their official definitions.
What is an economic operator?
An economic operator is the manufacturer, the importer, the authorised representative, the distributor, the fulfilment service provider or any other natural or legal person who is subject to obligations in relation to the manufacture of products, making them available on the market or putting them into service in accordance with the relevant Union harmonisation legislation.
What is a manufacturer?
A “manufacturer” means any natural or legal person* who designs or manufactures the product with the intention of making the finished product available for use under their name, marketing the name under their trademark.
What is an importer?
An importer refers to any natural or legal person established within the EU who places a product from a third (non-European) country on the European market.
What is a distributor?
Distributor refers to any natural or legal person in the supply chain, other than the manufacturer or the importer, who makes a product available on the European market.
What is a Fulfilment Service Provider?
A fulfillment service provider offers an end-to-end solution, taking products from warehouse shelves, packing them, addressing them and handing them to shippers, without owning the product. Parcel delivery services and any other postal services or freight transport services.
What is the New Legislative Framework?
This is a package of measures that was adopted in 2008 to improve the internal market for goods by strengthening the conditions for placing a wide range of products on the EU market.
What is Union Harmonisation Legislation?
This refers to the goal of the European Union to achieve uniformity in the laws of their member states, so that they can facilitate free trade and protect their citizens.
What is CE Marking?
The CE Marking is the manufacturer’s declaration that the product meets EU requirements for health and safety, as well as environmental protection. All products that must be CE marked will also require a European Authorised Representative.
What are Market Surveillance Authorities?
Market Surveillance Authorities are responsible for monitoring whether products meet the requirements of European safety laws.
*(Note the phrase “natural” or “legal” person. A “natural person” is an identifiable individual and a “legal person” is a corporate body, such as a limited company.)
You can always find definitions in the relevant European Legislation:

What is an Authorised Representative?
An Authorised Representative is any legal or natural person, established within the European Union, who has received a written approval from a manufacturer to act on its behalf in relation to specified tasks regarding the manufacturer's duties under the relevant harmonisation legislation.
The Authorised Representative acts as a liaison between the non-European manufacturer and a national authority within the EU. The Authorised Representative acts as a contact point for companies selling products to EU consumers, when the company doesn’t have any actual presence or an address anywhere in the European Union.
When there is an Authorised Representative Agreement signed between the AR and the manufacturer, the manufacturer can use the address of the AR on its packaging, which is required.
See here how the authorised representative is indicated on this packaging:

What is an EU Responsible Person?
Regulation (EC) No 1223/2009 on cosmetic products defines the Responsible Person (RP) as a natural or legal person withing the EU who is responsible for the product.
The responsible person must ensure compliance with the relevant obligations set out in the regulation.
What is an Amazon Responsible Person (ARP)?
Amazon uses ‘responsible person’ of 'Amazon Responsible Person' on their website, which they mean the EU-based economic operator. This can either be the manufacturer, importer or authorised representative. A ‘responsible person’ shall be located in the EU.
How Regulation (EU) 2019/1020 protects the internal market from unsafe products
So, why has this new Regulation been developed?
Over the last decade, ecommerce has become a thriving industry and globalisation and the internet have made it possible to sell all kinds of products all over the globe.
However, when products are sold internationally, it’s not always easy to see who the reseller is and where they are located. In the past, it has somewhat been a free-for-all when it comes to selling products across the border to consumers in the European Union.
Often, manufacturers are located outside the European Union, such as in China, the U.S. or the UK. When this happens, certain regulations must be followed for the product to be sold within the EU.
If a product is sold directly to the end user without establishing a known European economic operator, the EU has no control over the consumer protection and there is a risk of unsafe products entering the market.
Regulation (EU) 2019/1020 was established to address this problem. It will be effective from July 16th 2021 and it will require all sellers of products falling under the Regulation to have an economic operator within the EU.
The Regulation makes the internal market safer for European citizens. By strengthening the market surveillance of products, they improve the functioning of the European internal market.
This ensures that the products being sold are safe and of good quality, which reduces the risk of accidents, injuries and disastrous product recalls.
Prior to this Regulation, there was no effective mechanism for stopping the selling of unsafe products that are offered for sale online or shipped directlty to end users within the European Union.
This Regulation states rules and procedures for economic operators regarding products that are subject to certain Union harmonisation legislation.
In other words, every business from outside the EU selling products directly or indirectly should have either an importer or an authorised representative located in the EU.
Even if your business is based outside of the EU and doesn’t sell solely online, an Authorised Representative may be of added value. For example, you may not want your importer to be the one who has access to your full technical file, as it can often contain essential business knowledge.
And although an Autorised Representative has always been named in previous European product safety regulation, such as the CE directives, as someone who acts on behalf of the manufacturer in carrying out certain tasks, with the (EU) 2019/1020, the AR will be in the spotlight again.
Why Would I Need an Authorised Representative?
The Authorised Representative becomes a gatekeeper, protecting the market while helping manufacturers to comply with legal requirements of the European Union.
Countries being part of the European Union
An Authorised Representative has previously been described as an Economic Operator in other directives and regulations. This role has always encompassed certain tasks, depending on the types of laws, regulations and directives that apply to a particular product.
However, many businesses from outside the EU do not have an economic operator inside the EU. Instead, they sell their products directly to the end user, or via fulfillment service providers such as Amazon or Alibaba.
In these cases, there is no control over quality and it’s not clear who is responsible for the product.
Regulation (EU) 2019/1020 will make this more strictly regulated and will place more requirements on all products sold. Basically it means that you can’t sell a product on the EU market without the address of the European Economic Operator, such as the Authorised Representative, stated on the packaging anymore.
Due to Brexit, sellers from the UK will also now need an economic operator or an authorised representative within the EU. Vice versa, European sellers that sell products to the UK would also need an authorised representive based in the UK.
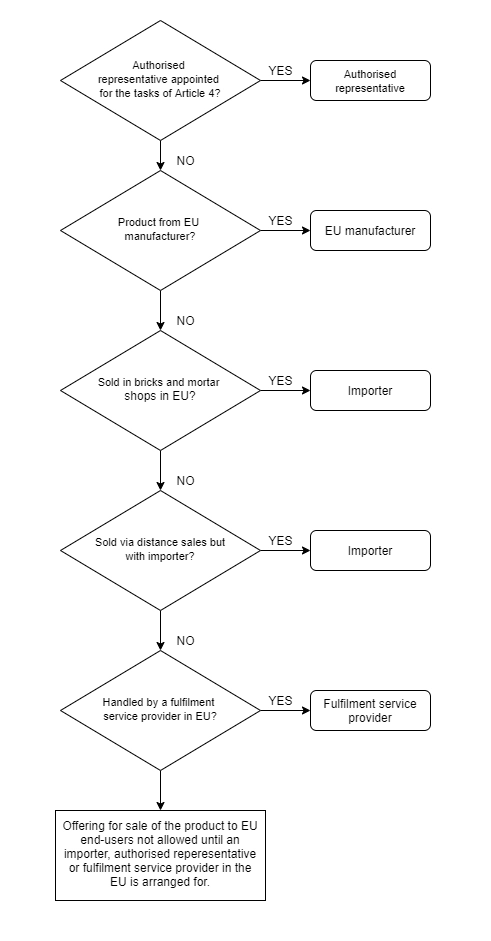
The economic operator in different supply chains (source)
What products need an Authorised Representative?
The following products need an economic operator, such as an Authorised Representative, established in the EU:
- Aerosol dispensers
- Agricultural and forestry vehicles
- Aircrafts
- Appliances burning gaseous fuels
- Batteries and accumulators
- Biocidal products
- Bottles used as measuring containers
- Cableway installations
- Chemicals
- Construction products
- Cosmetics
- Crystal Glass
- Detergents
- Electronic products
- Energy-related products (falling under Directive 2009/125/EC)
- Equipment for use outdoors emitting noise
- Explosives
- Fertilisers
- Fluorinated greenhouse gases
- Footwear
- Hot-water boilers fired with liquid or gaseous fuels
- Hydrogen-powered motor vehicles
- In vitro diagnostic medical devices
- Lifts and safety components for lifts
- Machinery
- Marine equipment
- Measuring instruments, such as most beverage glasses, pints etc.
- Medical devices
- Motor vehicles, trailers and systems
- Non-automatic weighing instruments
- Paints and varnishes
- Persistent organic pollutants
- Personal protective equipment, such as sunglasses, gloves, and face masks
- Packaging and packaging waste
- Petrol and diesel fuels
- Pre-packaged products that indicate the quantity units of weight or volume, not less than 5 g or 5 ml and not more than 10 kg or 10 l)
- Pressure equipment
- Products containing mercury
- Pyrotechnic articles, such as fire works
- Radio equipment
- Recreational craft and personal watercraft
- Simple pressure vessels
- Substances that deplete the ozone layer
- Textile products
- Tabacco products
- Toys
- Two- or three-wheel vehicles and quadricycles
- Tyres
DOWNLOAD THE FULL LIST OF LEGISLATION REQUIRING AN ECONOMIC OPERATOR
Does Regulation (EU) 2019/1020 apply when I sell products in the UK?
The short answer is no.
However, although the UK is not going to implement Regulation (EU) 2019/1020, they will still apply a market surveillance framework based on Decision EC 765/2008 (the New Legislative Framework).
The NLF requires importers that place goods on the UK market to be identified on the product or packaging.
So the requirement to have a UK based point of contact has actually been in place for some time, but has been widely ignored in the UK until now. Customs changes due to Brexit have brought the requirement to identify an ‘economic operator’ who is established within the UK in the spotlight again.
DO YOU NEED AN AUTHORISED REPRESENTATIVE?
We will support you in finding a suitable Authorised Representative to ensure you can sell your products on the European Market.
DO YOU NEED AN AUTHORISED REPRESENTATIVE?
We will support you in finding a suitable Authorised Representative to ensure you can sell your products on the European Market.
What are potential legal pitfalls when I would not have an economic operator within the EU?
At this stage, when you, as non-European manufacturer, do not have an economic operator, you might be wondering what would happen if you did not appoint an Authorised Representative.
What would be the consequences of this?
The truth is, failing to appoint an authorised representative may cause a great deal of issues for your company.
There are several checkpoints throughout the process of selling your product where it will be stalled if it does not have an address of an authorised representative associated with it.
Here are the points along the way where your product will be checked:
- If your product, machinery, device or equipment is shipped from outside of the EU and inspected by customs and it does not have an address of an economic operator, it will not be allowed to enter the EU.
- If your ecommerce product is (drop) shipped from outside of the EU and inspected by customs and it does not have an address of an economic operator, it will not be allowed to enter the EU.
- If it is not inspected by customs and it passes through, issues may arise when it gets to the Fulfilment Service Center. Marketplaces such as Amazon will check for the presence of an economic operator and if they don’t see an address, they will block the product.
- Yet another checkpoint will arise when your product is added by the seller to Amazon, or any other marketplace. Or more and more regulated products, Amazon requires a declaration of conformity, a photo of the packaging, a user guide and other important information. If the information doesn’t include the address of a European economic operator, the product will not be listed.
- Amazon (as well as any other marketplace) will regularly check their listed products to make sure the required address is there. If there is any doubt, they will request the address of the EO or AR. If you cannot supply it, they will take your products down. As with the (EU) 2019/1020 Regulation Fulfillment Centers will be seen as an economic operator en therefore will be responsible for certain tasks, most of them, if not all, will transfer the task to the seller who then needs to find an AR.
- If this happens, you will no longer be able to sell through Amazon FBA or other fulfillment centers, and you could possibly face issues with freight forwarders at some point.
Even more important than the compliance issues this will cause, there’s also the safety risk you will be creating. If your product and technical file has not been checked by an Authorized Representative or any other compliance vendor on completeness and compliance with EU requirements, you might sell an unsafe product.
For example, you might sell a toy that contains toxic chemicals, or an electrical product with a fault that can cause a fire. If this happens, you could be responsible and legally liable for the serious injury or even death of one of your customers.
Plus, it’s important to know that each Member State of the EU has market surveillance authorities who check the internal market for compliance with the requirements.
If they find out that you don’t have an authorised representative and don’t comply, you may be asked to do a recall of your product. Also, any products that are shipped in packaging without an EU Authorised Representative address can be seized and destroyed upon arrival.
You’ll have to deal with the financial loss of these products, and you’ll also have to compensate your customers who were waiting on an order that was seized and destroyed.

Product being checked at customs: no visible address? Then the package may be opened.
I am a Fulfillment Service Centre: What happens if I don’t appoint an Authorised Representative?
As a fulfillment centre, you will be seen as an economic operator for the products that you sell.
This means that you will have tasks such as verifying that the declaration of conformity and technical documentation have been drawn up, providing market authorities with all information and documentation necessary to demonstrate the conformity of a certain product and informing market surveillance authorities about products presenting a risk.
As a result of this, Fulfillment Service Centres, such as Amazon, partner up with companies providing AR services.
Do I need an Authorised Representative when I sell products in the UK?
As mentioned, also the UK requires to have an economic operator located within the UK when selling products on the UK market.
Manufacturers wishing to sell within the UK, but based outside the UK (including those based in the remaining 27 European countries), must have representation within the UK.
And although the UK is not going to implement Regulation (EU) 2019/1020, they will still apply a market surveillance framework based on Decision EC 765/2008 (the New Legislative Framework).
The NLF requires importers that place goods on the UK market to be identified on the product or packaging. Until December 31st, 2022 you can also provide the contact details on any accompanying document, rather than on the good itself.
So the requirement to have a UK based point of contact has actually been in place for some time, but has been widely ignored in the UK until now. Customs changes due to Brexit have brought the requirement to identify an ‘economic operator’ who is established within the UK in the spotlight again.
The UK guidance on placing manufactured goods on the market in the UK states the following:
Great Britain does not recognise authorised representatives and responsible persons based in the EU. If you need to (or choose to) use an authorised representative or responsible person, they will need to be based in the UK for products being placed on the GB market.

The UKCA (UK Conformity Assessed) marking is the UK product marking that is used for goods being placed on the market in Great Britain
How to avoid legal pitfalls and keep selling products on the European market
When you are a non-EU business and you want to sell products that fall under the scope of Regulation (EU) 2019/1020 on the European market, the only way to avoid legal pitfalls, is to have an importer or Authorised Representative within the EU.
When you are a non-UK business and you want to sell products that fall under the scope of Decision EC 765/2008 on the UK market, the only way to avoid legal pitfalls, is to have an importer or Authorised Representative within the UK.
For both scenario's, you should make sure to have the address of the importer or AR on the product, its packaging or an accompanying document.
What are the tasks of an authorised representative?
An Authorised Representative represents a non-European manufacturer and acts on its behalf in relation to ‘specified tasks’.
These tasks may differ, depending on the requirements that apply to the product. The tasks ogf the AR for several directives and regulations, including Regulation (EU) 2019/1020, are desribed in the below section.
An AR does not certify the products, but is responsible for reviewing the documentation provided by the manufacturer and optionally evaluating the certification procedures and safety of the products.
Some of the tasks an Authorised Representative may be responsible for include:
- Communicating with the Market Surveillance Authorities on behalf of the non-EU company.
- Verifying that the EU Declaration of Conformity or Declaration of Performance and technical documentation have been drawn up. (The Declaration of Conformity applies for CE products, such as toys, electrical equipment, machinery, medical devices, PPE, etc. The Declaration of Performance applies to Construction Products, according to Regulation (EU) No 305/2011)
- Carrying out a technical document review.
- Performing conformity assessments.
- Drawing up an authorised representative agreement and authorizing the non-EU company to use their address on their product packaging.
- Registering the product with the appropriate competent authority.
- Keeping a file of the product’s technical documents for potential inspection by EU regulators.
- Providing all documentation necessary to demonstrate the conformity of the product, if the Market Surveillance Authority requests information.
- Informing the Market Surveillance Authorities if the Authorised Representative has reason to believe that a product poses a risk.
- Cooperating with the authorities in the event that enforcement action is mounted against them.
- Performing any other tasks that are outlined in the written agreement between them and the manufacturer.
The official tasks and responsibilities of your AR will be laid out in the contract you sign, so make sure you read it thoroughly and understand it before proceeding.
Example of a declaration of conformity to comply with the Low Voltage Directive
What do specific directives and regulations say about the AR?
When it comes to authorised representatives, some particular industries will have more specific directives and regulations depending on the types of products sold. Let’s take a closer look into a few examples of these specific industries and what their regulations say.
Regulation (EU) 2019/1020 on market surveillance and compliance of products
Regulation (EU) 2019/1020 on market surveillance and compliance of products applies to all products listed in Annex I of the regulation. For an exact overview, see What Products Need an Authorised Representative?
However, if other directives and regulations regulate more specifically certain aspects of market surveillance and enforcement, these of course need to be taken into account.
So, without prejudice to any other obligations, the authorised representative, or other economic operator, must perform the following tasks:
- if these documents are required verifying that the EU declaration of conformity or declaration of performance and technical documentation are present.
- Providing market authorities with the information necessary to demonstrate the conformity of a product.
- Informing the surveillance authorities about product risks.
- Cooperate with the market surveillance authorities in case of non-compliance.
Toys (Toy Safety Directive)
Naturally, the children’s toy industry has a lot of safety regulations. This makes sense, as it’s important to make sure children aren’t playing with toys that are unsafe or could potentially cause harm.
The Toy Directive is part of CE Regulations and the AR is mentioned as being a possible economic operator in the directive.
A manufacturer may, by a written mandate, appoint an authorised representative.
If so, the AR can have the following tasks:
- Keep the declaration of conformity and the technical documentation at the disposal of national surveillance authorities for a period of 10 years
- Provide national authorities with the information and documentation necessary to demonstrate the conformity of a toy
- Cooperate with the competent national authorities
The following obligations are not allowed to be done by an authorised representative according to the toy directive:
- Making sure that a toy has been designed and manufactured in accordance with the requirements of the toy directive.
- Drawing up the the technical documentation
Electrical Equipment (Low Voltage Directive, EMC, RoHS, RED)
All electrical equipment sold on the European market should have an economic operator within the EU.
Electrical equipment can fall under the scope of several CE directives, such as the low voltage directive, EMC directive, RoHS directive and radio equipment directive.
EMC directive
According to the EMC directive, the AR may have the same tasks and limitations as the toy directive (see Toys (Toy Safety Directive)).
Additionally to these permitted tasks, the AR may affix the CE marking to each product that complies and can draw up the declaration of conformity.
If an AR has been mandated, the name of the AR shall be stated in the declaration of conformity.
Low voltage directive
Electrical equipment designed for use with a voltage rating of between 50 and 1 000 V for alternating current and between 75 and 1 500 V for direct current, falls under the low voltage directive.
The tasks and limitations of the authorised representative are the same as those of EMC directive. To summarise, the AR can:
- Keep the declaration of conformity and the technical documentation for a period of 10 years
- Provide national authorities with the information necessary to demonstrate the conformity of a product
- Cooperate with the competent national authorities
- Affix the CE marking to each product that complies
- Draw up the declaration of conformity.
The AR may not:
- Ensure that the product has been designed and manufactured in accordance with the safety objectives of the directive;
- Draw up the technical documentation.
Also for low voltage equipment: if an AR has been mandated, the name of the AR shall be stated in the declaration of conformity.
RoHS Directive
The RoHS Directive has a slightly different and broader way of formulating the tasks of the authorised representative.
First of all, manufacturers can appoint an authorised representative by written mandate, if they want.
The difference is, that the RoHS Directive specifically mentions that the mandate should describe the tasks that are appointed. The mandate shall allow the authorised representative to do at least the following:
- Maintain the DoC and the technical documentation for 10 years following the placing on the market of the EEE.
- Provide an authority with all the information necessary to demonstrate compliance.
- Cooperate with the competent national authorities.
The AR may not conduct the following:
- Drawing up of technical documentation;
- Ensure that the electrical equipment has been designed and manufactured in accordance with the requirements of the directive
Radio equipment
Equipment containing radio emitting components, such as WiFi, Bluetooth, Infra Red or GPS modules, must comply with the RED.
The tasks and limitations of the AR are identical to those of the RoHS directive.
If a Notified Body has been involved, the identification number of the notified body may be affixed by the authorised representative.
The AR may also lodge an application for the conformity assessment procedure. In that case, the application must include his name and address as well and he shall be notified of the decision of the NoBo and the name of the AR shall be stated in the declaration of conformity.
This product mentions just the name of the importer on the product itself. As the address is missing, the product is not compliant, unless the address is mentioned on the packaging.

WEEE Directive
The WEEE Directive is not part of the CE regulations and directives. However, it defines further tasks for the authorise represenative.
It states that all sellers of electronic products must be registered in the member state that they sell. This registration must be done through their authorised representative. This can can done through the online national registers of each member state.
Cosmetics
Regulation (EC) No 1223/2009 on cosmetic products uses 'responsible person' instead of authorised representative. Only cosmetic products for which a legal or natural RP within the EU is designated are allowed to be sold on the European market.
The responsible person must ensure compliance with the relevant obligations set out in the regulation and is fully responsible for the product.
The tasks of the responsible person are, amongst others:
- Ensure compliance with the relevant obligations set out in regulation (EC) No 1223/2009.
- Providing the SCCS with information on the product.
- Respond to requests form consumers to receive certain product-related information needed to make informed product choices.
- Providing competent authorities with a list of cosmetic products containing substances which have raised serious doubts in terms of safety.
- Making sure that the required information on the product is available for market authorities.
- Cooperate with these authorities upon their request on any action to eliminate the risks posed by cosmetic products which they have made available on the market.
- When there is a reason to believe that a product which they have placed on the market is not in conformity, the RP shall make sure to bring that product into conformity, withdraw it or recall it, as appropriate.
- Where the cosmetic product presents a risk to human health, the RP shall inform the competent national authorities.
- Identify the distributors to whom the cosmetic product is supplied.
- To make sure that the cosmetic product has undergone a safety assessment and that a cosmetic product safety report is set up in accordance with Annex I of the regulation.
Personal Protective Equipment
According to the PPE Regulation, the AR must do the tasks as specified in the written mandate received from the manufacturer. This should be at least keeping the EU declaration of conformity (DoC) and the technical documentation at the disposal of the national market surveillance authorities for 10 years after the PPE has been placed on the market, providing authorities with all the information and documentation necessary to demonstrate the conformity of the PPE and cooperating with the competent national authorities.
The CE marking and the identification number of the notified body by be affixed by the authorised representative, if not done by the NoBo body itself or by the manufacturer.
Besides keeping the DoC, the AR may also draw up the declaration of conformity.

Machinery
The machinery directive starts with mentioning that the authorised representative should receive a written mandate from the manufacturer to ‘perform on his behalf all or part of the obligations and formalities connected with the machinery directive.’
The AR may be appointed to do the following:
- Make sure that the technical file is available;
- Provide the necessary information, such as instructions;
- Make sure that the risk assessment has been conducted.
And optionally also:
- Make sure that the machinery meets the relevant essential health and safety requirements;
- Carry out the appropriate conformity assessment procedures;
- Draft the declaration of conformity and make sure that is included with the machinery;
- Affix the CE marking;
Watch this video on how to create compliant instructions for machinery:
Medical Equipment
The medical device regulation regulates tasks and responsibilities of AR’s most strictly. Here they can be responsible and held liable for defective devices.
For manufacturers of medical devices from outside the EU, the requirement to have an AR within the EU, already was obligatory. The AR plays an essential role in ensuring the compliance of the devices produced by those manufacturers and in serving as their contact person established in the EU.
The regulation clearly states that the authorised representative is legally liable for defective devices when a manufacturer established outside the EU has not ensured compliance. Together with the importer and the manufacturer, the authorised representative is jointly and severally liable.
The manufacturer from outside the EU must make sure that the AR has the necessary documentation permanently available.
Only when the manufacturer from outside designates a sole authorised representative, the device may be placed on the European market.
The AR must be officially mandated by the AR, who must accept the mandate in writing. The AR must perform all tasks specified in the mandate. A copy of the mandate must be provided to the authority, upon request.

As a minimum, the following tasks shall be appointed to the AR:
- Verify that the EU declaration of conformity and technical documentation have been drawn up;
- Verify that an appropriate conformity assessment procedure has been carried out by the manufacturer;
- Keep a copy of the technical documentation, the declaration of conformity and, if applicable, a copy of the relevant certificate, available;
- Comply with the registration obligations;
- Provide competent authorities with all the information and documentation necessary to demonstrate the conformity of a device;
- Forward to the manufacturer any request by a competent authority for samples, or access to a device and verify that the competent authority receives the samples or is given access to the device;
- Cooperate with the competent authorities on any preventive or corrective action taken to eliminate or, if that is not possible, mitigate the risks posed by devices;
- Inform the manufacturer about complaints and reports from healthcare professionals, patients and users about suspected incidents related to a device for which they have been designated;
- Terminate the mandate if the manufacturer acts contrary to its obligations. When terminated, the AR must shall immediately inform the competent authority of the Member State.
When there is a change of authorised representative this must be clearly defined in an agreement between the manufacturer, the outgoing authorised representative (if pzracticable) and the incoming authorised representative.
Construction products
Regulation (EU) No 305/2011 for the marketing of construction products allows the appointment of an authorised representative (by written mandate) for at least the following tasks:
- Keep the declaration of performance and the technical documentation for 10 years.
- Provide the market surveillance authority with all the information required to demonstrate the conformity of the construction product with the declaration of performance and compliance with other applicable requirements in the construction products regulation
- Upon request, cooperate with the competent national authorities.
The AR is not allowed to draw up the technical documentation.
Article 43 states that a notified body carrying out the conformity assessment can never have the role of the authorised representative.
Also, the regulation on construction products contains a draft for the declaration of conformity where the authorised representative should be indicated.

REACH Regulation (EC) No 1907/2006
REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals.
REACH applies to all chemical substances, such as those used in industrial processes, but also those in our day-to-day lives (e.g. in clothes, paint, cleaning products, furniture etc. Therefore, the regulation has an impact on most companies selling products on the European Market.
Also products that fall under the scope of REACH need to comply with Regulation (EU) 2019/1020 and thus need an authorised representative.
The REACH Regulation states the following:
In addition, if the supplier is not located in the Member State where the substance or mixture is placed on the market and he has nominated a responsible person for that Member State, a full address and telephone number for that responsible person shall be given.
The REACH Regulations mentions the 'Only Respresentative'. The Only Respresentative is a natural or legal person established in the EU appointed by the non-European manufacturer (who manufacturs regulated substances, mixtures or articles) to fulfil the obligations on importers under the REACH Regulation.
Is an Authorised Representative legally responsible for defective products?
If your product is defective, who is legally responsible?
The answer to this depends on the directive and the particular industry you are in.
For example, according to most CE Regulations and Directives, such as the Toy Safety Directive, the Low Voltage Directive and the EMC Directive, the Authorised Representative is not liable.
In most cases, the manufacturer is responsible and liable for the product. When the product is being imported, the importer plays a key role guaranteeing the compliance of imported products.
Particularly when an importer (or a distributor) markets a product under its own name, they take over the manufacturer’s responsibilities and are liable for defective products.
In some cases, also the Authorised Representative is liable.
For example, according to Regulation (EU) 2017/745 on Medical Devices, the AR is liable for defective devices in the event that a manufacturer from outside the EU has not complied with its general obligations.
In the Medical Device industry, the AR must terminate the contract with the manufacturer if the manufacturer has not complied with their obligations. The authorised representative is jointly and severally liable with the importer and the manufacturer.
So, it’s worth taking some time to research your particular industry to find out whether or not the Authorised Representative will be the one liable if your products are faulty or defective.
For medical devices, an AR is liable for defective products and non-compliance
Can I use my distributor as my Authorised Representative?
If you already have a distributor, it is possible to use them as your authorised representative? Are there any issues with this?
It is possible to use your distributor as your authorised representative, and you might be tempted to save money by doing so. Companies that sell Class I devices who only intend to sell in a few countries with a single distributor could save on the cost of appointing an AR.
However, there are some things you should consider before you decide to do so.
Here are some of the downsides of using your distributor as your authorised representative:
- Your distributor’s name and address will need to be printed on all of your packaging. So, if you decide to change distributors, you’ll need to reprint all labels, packaging and manuals. Plus, you’ll need to figure out what to do about the products already on the market that have your old distributor’s name on them.
- Since a distributor is focused on the sale and marketing of your devices (rather than regulations), they may not necessarily keep abreast of any changes in European laws and guidelines and adjust accordingly.
- Your distributor will have access to your Technical File, which includes proprietary information.
- If the Market Authorities happen to question an incident concerning one of your products, can you be sure that they will defend your company? Or, will they protect their own interests?
Since the annual cost of an Authorised Representative is usually only around $3,500, it may not be worth the risks and downfalls of using your distributor to cover this role.
5 Steps To Choosing a European Authorised Representative
How can you find an Authorised Representative? There are several different companies available offering this service in the EU. The key is to find the right company for you and make sure the service they offer is the right fit for your business.
When it comes to choosing an Authorised Representative, the process can be broken down into five simple steps.
Step #1: Find an AR
Compare a few different options, as every AR will provide slightly different services and will charge different fees. Many will offer to custom-tailor a contract for you, so you can find a representative who will meet your needs.
It’s a good idea to look for an AR who has experience with a wide range of products and devices. They may not have expert knowledge of the product you are selling, but they will be able to help guide you through the regulations and requirements that apply to your device.
You should also look for an Authorised Representative who has been around for a few years. This means they are less likely to go out of business and you won’t have to pay to reprint all of your packaging.
Also, a company with several years of experience in the business will have stronger connections and relationships with Competent Authorities, which could potentially work to your advantage if any problems arise.
Step #2: Send a Declaration of Conformity and a Technical File to the AR
The next step is to prepare the required paperwork and send it to your AR. You’ll need to send the Technical File and a Declaration of Conformity, plus any other paperwork that might be required depending on your industry.
Your AR can let you know the specifics of what is required. For example, if you are in the medical device industry, your AR will need to verify that you are complying with the registration of the Unique Device Identification, according to article 27. Also, they will need to check that the registration of the device has been performed according to article 29.
Step #3: The AR Verifies that DoC and Technical File Have Been Drawn Up
During the next step, the AR will verify that the DoC and the Technical File have been drawn up. If they require any extra info from you during this stage, they will let you know.
The AR will keep the documentation on file for the amount of time required by your particular industry. For example, for medical devices the files must be kept for at least 10 years and for implantable devices, it is 15 years.
If you are working with an Authorised Representative who works with multiple clients, make sure they have a clear and organised system for Record Retention. This will help make sure that they are not at risk of losing any information, or providing the wrong version of documents.
Some Authorised Representatives will offer you to conduct a gap analysis as well to check if the sent technical file is not only present, but also is complete, authentic and complies with the requirements.
Step #4: Sign AR Agreement
The next step is to sign an agreement with your Authorised Representative. The contract will outline exactly what the authorised representative will be responsible for and what you need to do to uphold your compliance.
Step #5: Use the Address of the AR On Product Packaging
Finally, the address of the AR must be displayed on all your product packaging, product label or any other accompanying document. It should include the manufacturer’s name and address and the name and address of the European Authorised Representative.
The “label” is defined as either the packaging of each unit or the packaging of multiple devices.
You’ll also need to include the CE Marking and the country where the product is produced. The product packaging must include user instructions and safety instructions, or it must indicate that these instructions are present within the packaging.
You may also need to add a warning logo if the product is not suited for children younger than a certain age, as well as other information such as whether the package is recyclable or should not be disposed of with the domestic waste. Keep in mind that there may be other requirements for specific products, depending on the applicable directives.
Companies offering Authorised Representative services
24hour-AR
24hour-AR is a compliance company with over 25 years of experience in the field of product safety. 24hour-AR acts as a legal entity representing non-European Union businesses from outside the EU and supporting them with EU compliance requirements, so they can do business easily anywhere in the EU. Their services help avoiding legal pitfalls by allowing to place their EU address on your product.
Where most companies take two weeks or more to act as an Authorised Representative, 24hour-AR promises a signed AR agreement in just 24 hours or you'll get a 50% discount. They focus on all products, except medical devices.
Next to AR services, the company also offers legal consultancy, full CE Marking support and technical communication services. With this, 24hour-AR is a full-service compliance partner. The annual fee for the service starts at 699 Euro.
Address: Storgatan 51, 903 26 Umeå, Sweden - https://24hour-ar.com/
ProductIP
ProductIP is a compliance firm in the Netherlands. The company helps businesses that are looking to expand to the EU to comply with the legal requirements. They have integrated their services into a self-built platform, in which you must store your technical file. Some legal knowledge is required in order to use the system.
According to their website, customers that want to use AR services need to provide a valid business license/company registration document, product liability insurance and an EU AR Agreement.
The annual fee for the service is 2,000 Euro and they charge 225 Euro per hour, for handling any issues related to their AR responsibilities, such as communication with the market surveillance authorities. They request a deposit of 1,800 Euro, which covers the first 8 hours of work.
Address: Rubensstraat 211, 6717 VE, Ede, The Netherlands
AR Experts
AR Experts started as a spin-off in 2020 in the Netherlands, and offers its services as an AR for exporters who are looking to sell their products on the European market.
AR Experts has a strong focus on toys, electronic products, and medical devices. Prices are available upon request. According to their website, they can register businesses as their Authorised Representative within two weeks.
Address: Amerlandseweg 7, 3621 ZC Breukelen, The Netherlands.
Qserve Group
Another player from the Netherlands is Qserve Group. The company was founded in 1998 and claims to be an expert in providing consulting services for medical device manufacturers. They specialise in legal consultation, quality control, clinical trials, and educational training.
For manufacturers of medical devices who are looking for an AR in the EU, Qserve offers to use their address, communicate with the authorities, maintain the technical file and provide education.
Address: Utrechtseweg 310, 6812 AR Arnhem, The Netherlands,.
Prevent your products from being blocked from entry into the EU
Complying with European regulations can be challenging, but it’s incredibly important. You’ll need to find an Authorised Representative with a strong network and good connections in Europe, who stays updated and informed in regulatory affairs and who makes serving your representation needs an important priority.
European authorities will continue to tighten regulations and be more vigilant with the products entering the market. Choosing an Authorised Representative who can help you stay compliant is more important than ever and will be essential for the future of your company. It helps you to avoid legal pitfalls and ensures that you can keep selling your products on the European or UK market.
We can help you find an AR that suits your needs. If you want to know more, contact us today.
 |
Ferry Vermeulen is a technical communication expert and director at INSTRKTIV. It's Ferry’s mission to create digital user instructions for all products in the world. Listen to the INSTRKTIV podcast on Spotify or read one of his latest blog articles. Linkedin I Spotify I YouTube I Facebook I Twitter |
DO YOU NEED AN AUTHORISED REPRESENTATIVE?
We will support you in finding a suitable Authorised Representative to ensure you can sell your products on the European Market.

