Declaration of Conformity Definition, Templates and Guide
Law and Legislation
- Scroll to the bottom of this page to download our free 27 templates -
The EU Declaration of Conformity is a necessary document for CE marked products. If you market your own products, sell on ecommerce platforms such as Amazon, export to the EU, write user manuals, or work in R&D, you'll likely need to deal with a declaration of conformity (DoC). This post will teach you how to draw up a compliant DoC quickly and easily using the DoC-Method and templates provided. Before we get into the guide and templates, please let me explain to you the definition of a declaration of conformity.
What is a declaration of conformity?
The declaration of conformity is a legal document that needs to be signed by the manufacturer. With the document the manufacturer formally declares the compliance of a particular product, that falls within the scope of CE marking, with the essential health and safety requirements of the relevant product safety directives.
The EU Declaration of Conformity is a legal document that is mandatory for products that fall within the scope of CE marking. Depending on the kind of product, the declaration can be stored electronically in the technical file, or must be provided with the product.
By signing the declaration of conformity, the manufacturer or importer (an importer is legally seen as the manufacturer) takes full responsibility and liability for the product’s compliance with the applicable legislation.
With a properly drawn up declaration and an affixed CE marking on the product, buyers, end-users and market surveillance authorities will assume that the product is compliant with the CE directives and harmonised standards mentioned in the declaration of conformity.
Signing the DoC is the last step in the CE marking process.
When signing the declaration, one should be sure that the other steps have been completed successfully so that the product fully complies. See this article and podcast about how to CE mark a product.
Example of a Declaration of Conformity
Depending on the directive(s) that apply to a product, different requirements on the content of the declaration may apply. The below example displays an example of a declaration conformity for a product that needs to comply with the Low Voltage Directive.

Example of a Declaration of Conformity. Scroll to the bottom of this page to download our 27 templates.
Who can sign the declaration of conformity?
According to the CE marking directives, every manufacturer or duly authorised representative of the manufacturer, based in any country within the European Economic Area (EEA), is obligated to draw up a Declaration of Conformity. This is in compliance with the conformity assessment procedure. This declaration must be issued prior to the placement of products on the market in Europe.
The DoC will never be issued by a Notified Body or a test house. Also, when a product is imported from a non-EU country, it is the responsibility of the importer to make sure the product complies with all legal requirements and also that the technical file (which included the declaration) is correct and available.
Distributors don’t need to draw up the declaration but must verify that the manufacturer or importer has taken the required measures. Only if distributors (or importers) sell the product under their own brand name, should they take over the manufacturer’s responsibility and draw up and sign the declaration.
Besides the (legal) manufacturer, the declaration can be signed by the authorised representative of the manufacturer. The authorised representative is a natural or legal person established in the European Economic Area who, explicitly designated by the manufacturer in a non-EU country, acts on his behalf in carrying out certain tasks regarding the CE marking. Thus, in essence, the signatory should also be the person to be held accountable for any invalidity regarding CE compliance.
Which products need a declaration?
All products that fall within the scope of CE marking must have a declaration of conformity or a variant of it (e.g. a declaration of incorporation for partly completed machinery or declaration of performance for construction products). There are CE directives for the following product groups:
- Cableway Installations
- Construction Products
- Electrical Equipment
- Energy-Related Products
- Equipment Used in Explosive Atmospheres
- Explosives
- Gas Appliances
- Implantable Medical Devices
- In Vitro Diagnostic Medical Devices
- Lifts
- Low Voltage Equipment
- Machinery
- Measuring Instruments
- Medical Devices
- Outdoor Equipment with Noise Emission
- Personal Protective Equipment
- Pressure Equipment
- Pressure Vessels
- Products containing Hazardous Substances
- Pyrotechnical Articles
- Radio Equipment
- Recreational Craft
- Toys
- Water Boilers
- Weighing Instruments
To verify if your product falls under the scope of CE marking, you should verify whether the directive applies to your product in each directive. To do so, you should check the scope of each directive, as well as the definitions and exceptions.
When a product falls under more than one directive, in most cases a single Declaration will be issued declaring conformity to more than one directive. Alternatively, there may be more than one Declaration for each directive product falls under.
In cases where a CE marked product is incorporated in another product (for example a lock in a machine), the Declaration for the main product may only declare the conformity of the main product. Thus, the Declarations for the CE marked parts of the main product should be included in a technical file for the main product.
What if I don’t have a declaration?
When you trade a product for which a declaration of conformity is required and you do not have a (compliant) one, legally you are not allowed to sell your product. In order to place a product on the European market, make sure you draw up a compliant declaration. When doing so, always make sure that your product complies with the relevant legislation. Nevertheless, you are declaring compliance and taking all responsibility of the product by signing the document.
Platforms such as Amazon also require a DoC for certain product groups, such as toys, batteries, chargers, lighting and kitchen equipment. This is part of Amazon’s pre-approval process. By doing so, Amazon takes the responsibility to ensure that only safe products are sold on their platform. As many sellers are not aware of their legal responsibilities, Amazon wants them to be aware of the fact that they, as the seller, are fully responsible for the product. If you can’t submit a (compliant) declaration, you can’t sell your product on the platform or you could even get banned,
When importing products from outside the EU, Customs randomly inspect products. Often they will ask you to submit a declaration of conformity. If you can’t provide them with one, they will not release your product.
What should be in the declaration of conformity?
Though the basic elements of a Declaration are common throughout the most directives, the requirements on the content of the DoC can differ slightly per directive. However, almost all of them must include the following:
- Name and full business address of the manufacturer or the authorized representative;
- Some kind of identification that allows the product’s traceability. This could be a serial number, model or type identification;
- A statement, stating that the manufacturer takes full responsibility;
- If applicable, the name, address and identification number of the Notified Body which carried out the conformity assessment procedure;
- The relevant legislation with which the product complies, as well as the harmonised standards or industry standards used;
- Name and signature of the manufacturer;
- The date the declaration was issued.
Many directives have additional requirements for the content of the declaration. As an example, the Machinery Directive requires that the declaration must include the name and address of the location of the technical file of the product.
When to Provide A Declaration Of Conformity
The Declaration of Conformity should be part of the technical file and made available upon request of market authorities. Only in some cases, it should be provided with the product.
Do you need to translate your declaration of conformity?
Most directives, such as the Radio Equipment Directive and the Low Voltage Directive, require that the declaration should be translated into the language or languages required by the Member State in which the product is placed on the market. Older directives often do not state this very clearly, but a translation is always recommended as market surveillance authorities can always require to submit a translated declaration of conformity.
Should you provide the declaration with the product?
Some directives, such as the Machinery Directive, require the DoC to be accompanied along with the product/machinery. The Radio Equipment Directive gives the possibility to provide a copy of the full EU declaration of conformity or a simplified EU declaration of conformity. For quite a few directives it is allowed to have the declaration available in the technical file only.
What are the synonyms and variations of the EC declaration of conformity?
CE certificate
Although it is not the officially used name, the EC declaration of conformity is sometimes also called a CE certificate.
CE declaration of conformity
A CE declaration does not exist formally.
CE Statement
Although it is not the officially used name, the EC declaration of conformity is sometimes also called a CE Statement.
Declaration of incorporation
Partly completed machinery that is first placed on the market will not need CE marking. However, it must be accompanied by a declaration of incorporation instead of a declaration of conformity.
Declaration of performance
Instead of a declaration of conformity, construction products need to be accompanied by a declaration of performance. This declaration provides information about the performance of a product.
EC declaration of conformity
Often used name for the declaration of conformity, although not used by the European Commission.
EU declaration of conformity
This is an official variation of the declaration of conformity. Also used on the website of the European Commission.
What is the difference between a certificate of conformity and a declaration of conformity?
A certificate of conformity is a declaration for vehicle with which the manufacturer declares that the vehicle complies with the given approved type.
How do I draw up a Declaration of Conformity?
Whether you are selling on Amazon, importing or manufacturing products or are an entrepreneur, technical writer, QA manager or translator, the chances are good that you will have to deal with a declaration of conformity (DoC).
These legal documents that are the last step in the process of CE-marking, need to be integrated into the user manual or need to be included with the product you (or your company) sell, or in the technical file.
Someone has to draw up the DoC. And if there is no official safety expert, the company often relies on the technical author or product engineer. And if there is no technical writer or product engineer you might be responsible for the DoC yourself!
Do you struggle with drawing up a DoC?
Well,.. not any more!
Read on to learn how to create a compliant DoC for your specific product.
Being involved in both product development and technical communication, I have always been interested in CE-marking. That's why I got more and more involved in the CE marking process, risk analysis, technical files and compiling DoCs.
Before I used the method that I describe in this post, I spent ages on the internet, finding the right information on what to include in the DoC and what not. And at the end, I still wasn't sure!
That's why I structured this way of working and 'developed' the Declaration-of-Conformity-Template Method.
By using the DoC-Template-Method:
- I do not need to crawl through the maze of those ever-changing EU websites (however I think I am one of the few who actually finds this interesting).
- It is very clear what information should be included in the EU-Declaration of Conformity for a certain product.
- I always have a good example of a DoC for my product group.
And the best part? You can do exactly the same! The only thing you need to do is to follow the steps as described below. It is a method that includes good examples and templates for each product group that requires a DoC. So if you want to draw up compliant DoCs, keep on reading!
Let’s break it down into actionable steps.
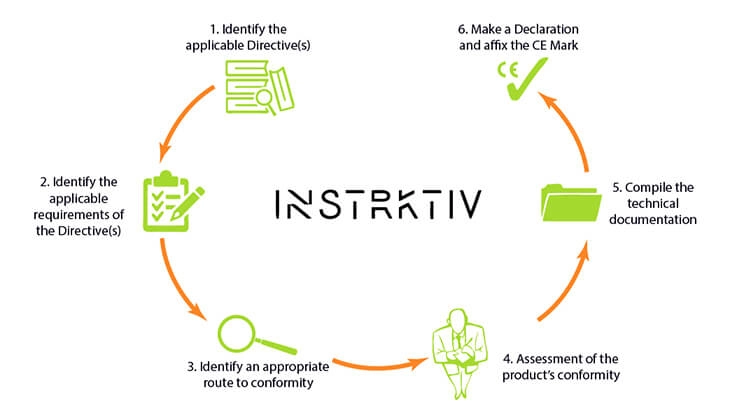
WHAT DOES THE CE MARKING PROCESS LOOK LIKE?
Overview of what all manufacturers, importers and distributors should do to obtain CE marking
Just 4 steps bring you to a compliant DoC
There are just 4 steps that make up The Declaration-of-Conformity-Template-method:
- Go to the EU site. Select your product group(s).
- Find all the information about the DoC in the directive you need
- Download the correct template(s) and draw up your DoC
- Check if you have met all the requirements
Step #1 Go to the EU site and find your directives
There are 25 product groups that require CE-marking and thus a DoC. By the way, for construction products the document is called a Declaration of Performance and for partly completed machinery a Declaration of Incorporation. For the sake of simplicity, in this article I will call all of them declaration of conformity.
If you have a product that fits into one of those product groups, then you have to draw up a DoC.
Many products need to comply with more than one directive. If this is the case for your product, all steps described below need to be performed for each applicable directive.
The requirements for the contents of the DoC can mostly also be found in the applicable directive. Most directives have their own specifications regarding the DoC and very few directives have exactly the same requirements.
Sometimes you will not find any requirements regarding the DoC in a directive (e.g. the Directive 90/385/EEC on Active Implantable Medical Devices does not give any requirements). For those directives the harmonised standard EN-ISO/IEC 17050-1:2014 Supplier's declaration of conformity -- Part 1: General requirements can be used. Because, above all, compliance with harmonised standards provides a presumption of conformity with the directive.
But if a directive has its own specific requirements, like the Machinery Directive or the Radio Equipment Directive, the EN-ISO/IEC 17050-1:2014 can be used as a basis, but it should be enhanced or modified according to the additional requirements as set out in the directive.
Another thing to be aware of is that directives change now and again. And if they change, there is a big chance that the requirements of the DoC change as well.
Now don’t be afraid! I have done all the hard work for you. But because it is your product you’re dealing with, I would like you to also have a close look yourself. This will be done in step 2.
To find the directives that apply to your product:
- Go to the website of the European Commission
- Click on the product group that applies to your product. Click on the link that appears.
-> A new page appears with Information about the product group. - Click on the link to open the directive (e.g. the link Directive 2006/42/EC on Machinery).
-> A page with links to the different languages and formats of the directive opens. - Select your language and format (e.g. English and pdf).
-> The directive opens
Step #2 Find all information about the DoC in the directive
To find all requirements and other relevant information:
- Press ctrl+f and type
declaration of conformity
-> the number of hits is shown. Note: for construction products you should typedeclaration of performance.For partly completed machinery you should typedeclaration of incorporation. - Create a table with two columns in a document or spreadsheet.
- Copy/paste in the left column the number of the article and in the right column the text which contains “declaration of conformity”. Read the text carefully. Copy/paste all other hits to the table.
Example: DIRECTIVE 2014/28/EU - explosives for civil uses
|
Article no. |
Text |
|
24 |
To ensure effective access to information for market surveillance purposes, the information required to identify all applicable Union acts should be available in a single EU declaration of conformity. In order to reduce the administrative burden on economic operators, that single EU declaration of conformity may be a dossier made up of relevant individual declarations of conformity. |
|
Article 5.2 |
Where compliance of an explosive with the applicable requirements has been demonstrated by that procedure, manufacturers shall draw up an EU declaration of conformity and affix the CE marking. |
You have now created an overview of all relevant information regarding the DoC. Next to the specific requirements on the content, this can also be information about the requirements whether to translate the DoC or not, to deliver the document with the product, or to draw up a single document for all applicable directives.
Step #3. Download the correct template and draw up your DoC
Now you are aware of the requirements, it’s time to draw up the DoC for your product.
In order to do draw up your DoC:
- Scroll down to the bottom of this page.
- Select correct template(s). The pdf will be sent to the email address provided.
- Draw up your own DoC of conformity, based on the template provided.
Step # 4. Check if you have met all other requirements
Now you have created your DoC that meets all content requirements, it is time to check the other requirements. For this, the document you created in step #2 can be used as a checklist.
Action: check the document you created for other important information about translating the DoC, the medium to be used et cetera.
That’s all!
Some facts about the declaration of conformity
- A DoC is sometimes also called a CE declaration of conformity or EC declaration of conformity
- DoC vs certificate of conformity: A DoC is not the same as a Certificate of Conformity (CoC). A CoC (or type approval) is granted by a Notified Body to a product that meets a minimum set of regulatory, technical and safety requirements.
If you gained some value from my post, I’d appreciate a comment or social media share!
 |
Ferry Vermeulen is a technical communication and compliance expert. He also is a parttime trainer at the Dutch standardisation institute (NEN). Listen to the INSTRKTIV podcast on Spotify or read one of his latest blog articles. Linkedin I Spotify I YouTube I Facebook I Twitter |
DO YOU WANT TO KNOW HOW YOU CAN CREATE A COMPLIANT USER MANUAL FOR THE EU?
A step-by-step approach to develop CE-compliant user instructions and avoid legal pitfalls.
Download Our Free Declaration of Conformity Templates Here:
-
2000/14/EC Noise emission

-
(EU) 2016/424 Cableway inst.

-
2006/42/EC Machinery

-
2009/125/EC Ecodesign

-
(EU) 2016/426 Gas appliances

-
2009/48/EC Toys

-
2011/65/EU RoHS

-
2013/29/EU Pyrotech. articles

-
92/42/EEC Hot water boilers

-
2014/28/EU Explosives

-
2014/29/EU Pressure vessels

-
2014/30/EU EMC

-
2014/31/EU Weighing instr.

-
2014/32/EU Measuring instr.

-
2014/33/EU Lifts

-
2014/34/EU ATEX

-
2014/35/EU LVD

-
2014/53/EU Radio equipm.

-
(EU) 2017/746 In-vitro diagnostic

-
2013/53/EU Recreational craft

-
2014/68/EU Pressure equipment

-
(EU) 2019/945 Unmanned aircrafts

-
(EU) No 305/2011 Constr. prod.

-
(EU) 2017/745 Med. devices

-
(EU) 2016/425 PPE
