The Ultimate Guide on What Your Technical File Should Contain
26/02/2023 Compliance
This article provides 101 examples to help you compile your technical file. It not only provides examples to comply with the various CE directives and regulations, but also for compliance with other directives, such as the general product safety directive (GPSD) and the regulation on the registration, evaluation, authorisation and restriction of chemicals (REACH). After reading this article, I hope you have sufficient examples to help you to compile your technical file yourself.
What is a technical file or technical documentation?
The technical file is the set of documents that describe a product, device or machine and demonstrate that the product is designed in accordance with the requirements of the relevant directives and regulations.
All products covered by the CE marking must have a technical file containing the information to demonstrate that the product complies with the CE directives and regulations. The content of the technical file is determined by the relevant directives and regulations: the technical file of a machine contains the components as described in the machinery directive, and the technical file of toys as laid down in the toys directive. The compilation of the technical file is step 5 in the CE marking process.
But non-CE products should also have a technical file. How else would you prove compliance with regulations and directives such as the packaging directive, REACH and the GPSD?
What is in a technical file?
When a product falls under more than one directive, the technical file consists of the sum of the requirements for the file as stipulated in all relevant directives and regulations. The file may also include documents to demonstrate compliance with non-CE legislation, such as the REACH regulation, the WEEE directive or even information on the use of intellectual property.
What is a technical file for the MDR?
A technical file according to the medical device regulation (EU) 2017/745, also known as the MDR, contains all information about the medical device (such as its claims, clinical evaluations, reports and design information) to prove compliance with the regulation. It is one of the most complicated technical files, as many technical documents may be required.
At a minimum, a technical file for medical devices should contain:
- A device description and specification section, including the UDI number.
- Labelling information and a copy of the instructions for use (IFU).
- Design and manufacturing information.
- Risk management information according to ISO 14971.
- An overview of the General Safety and Performance Requirements (GSPR).
- Verification and validation information.
- Post-market surveillance (PMS) information.
What is an example of a technical document?
We should differentiate between two things: a technical file and a technical document. The technical file is structured in a certain way, depending on the relevant legislation. The technical file may contain several technical documents, such as test reports, the declaration of conformity and a copy of the user instructions.
As it is impossible to provide a single example of a technical document, this article provides some examples for the most common technical documents.
Technical file checklist
Although the relevant legislation determines what should be in a technical file, and thus the technical file of a toy is different from that of machinery, there are certain elements that overlap. This checklist provides a high level overview of what should be included in the the technical file for most products:
- Product design information
- General description
- Intended use
- Model, type, batch or serial number
- Pictures
- Bill of materials
- Mechanical drawings or exploded view
- Applied standards and specifications
- Design calculations, inspections and examinations
- Risk assessment
- User instructions, markings, labels & information
- Packaging information
- Test reports
- Declarations
- Quality assurance information
- Product registration information
These templates contain a structure for specific product groups.
Examples of what could be required for your technical files
Product design information
Each technical file should contain information on the design of the product. A product can be described using words or using drawings. The sections below give an overview of the most common ways to describe a product.
General description
Providing a general description is mandatory under the machinery directive. But many other directives also require that a detailed description of the design and manufacture should be included. It is up to the person responsible for the product and technical file how to fulfil this requirement.
This is an anonymised example of the general description of a piece of machinery:
The [product name], with provisions for Ethernet and USB communications, is used to test the airtightness of your food packages at speeds reaching 80 packages per minute (depending on the package size). The measuring process takes place in the machine immediately after sealing the packages in the packaging line. This prevents the packages from being rejected further down the supply chain. After the predefined food packages are put in the measuring chamber, vacuum is applied and the pressure decay in the chamber is measured. If a leak is detected, the food package concerned is immediately guided to the rejection area and taken out of the production line.
This is an anonymised example of the general description of an electrical product:
The [product name] is an AC charging station that you can use to supply electricity to an electrical vehicle. The [product name] offers tailor-made, intelligent and network charging solutions for your company or home. The EVSE can connect to the internet via GSM, WiFi or LAN.
But a product or machine can also be described by means of illustrations, indicating its main components:
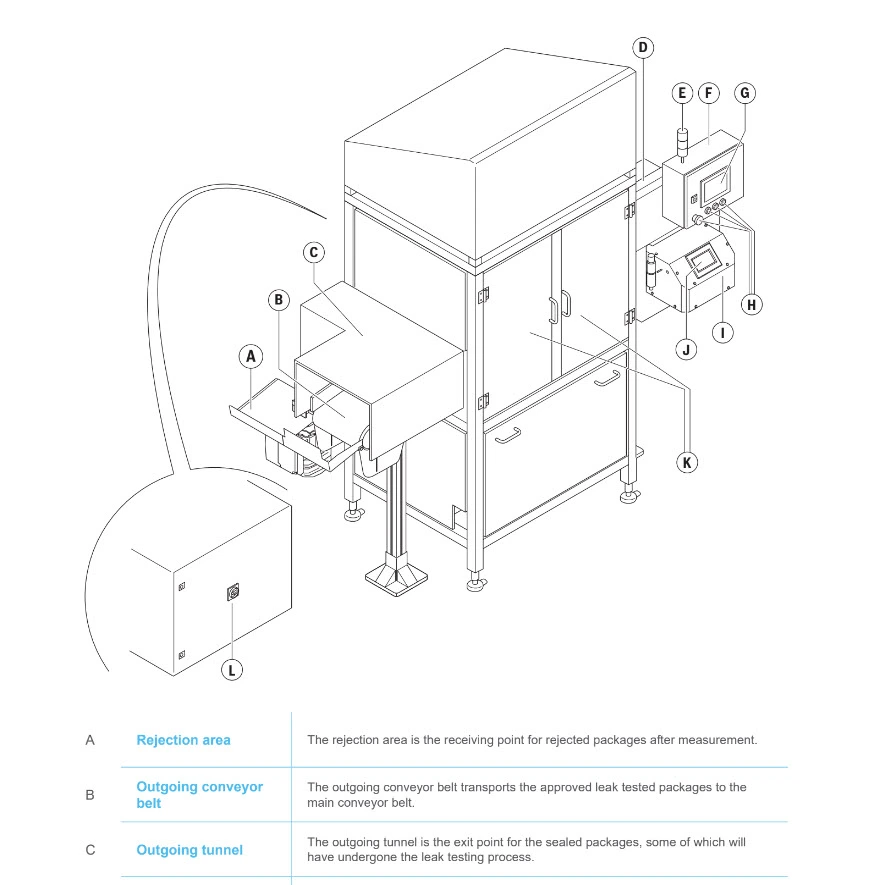
Declaration of product identity
The declaration of product identity (DoPI) is a declaration with which you declare that the product that you are selling under your own name is the same as the product mentioned in test documents.
Especially when products are purchased outside the EU, the non-EU OEM manufacturer conducts product testing. These test reports mostly mention the original product's name and not the name under which you market your product. If this is the case, you need to declare that the product to which the test report applies is the same product that you sell under your own name.
Here is a template that you can use to create your own declaration of product identity:
Declaration of Product Identity
We (supplier) hereby confirm that the following product as mentioned in the test report:
…………………………………….
is identical to the following product which is placed on the market:
…………………………………….
Confirmed by:
Intended use
The intended use can be described as an exhaustive range of functions or foreseen applications defined and designed by the supplier of the product.
The intended use describes the purpose of the product, the reason why it is designed and what it should do or solve. It also determines which directives apply to the product.
This is an anonymised example of a general description of machinery:
The [machinery] is intended to be used for:
- Mechanically brushing the outer glass and gutters of greenhouses.
- Applying a temporary protective layer to the roof by means of optional spray masts.
The [machinery] is not intended to be used:
- As a device to move persons or objects.
- To conduct repairs to the greenhouse roof.
- As a snowplough.
- At temperatures lower than 0.5°C (33°F).
- At wind speeds more than 11 m/s (25 mph).
Only use the machinery within the specified performance limits. The machinery must only be used according to the instructions as described in the documents accompanying the machine. All use other than as described in this manual is seen as unintended use. The machinery shall be used with original accessories and components only.
This is an anonymised example of the intended use of an electrical product:
The [product name] is intended for the AC charging of electric vehicles. The [product name] is intended for indoor or outdoor use. Only use the [product name] with accessories that the manufacturer provides or that obey the local rules.The [product name] AC input is intended for a hardwired installation that complies with the applicable national regulations.
Model, type, batch or serial number
It’s hard to miss this one when you dive into product safety legislation. You will find this requirement in basically every directive.
It is required that you mark the product with a type, batch, serial or model number or other element allowing the product’s identification. If market authorities have reason to believe that a product presents a risk, they will request the technical file of that product.
For that reason, it’s crucial that the technical file contains the same identification of the product as the product’s marking does. The identification of a product shall be carried through consistently in all documentation, labelling, packaging, etc.
An advantage of adding a product identifier is that it may limit the quantity of product that needs to be withdrawn from the market. For example, in the event that only type CS20M of an electric scale presents a risk, then type CS40M will not need to be withdrawn.
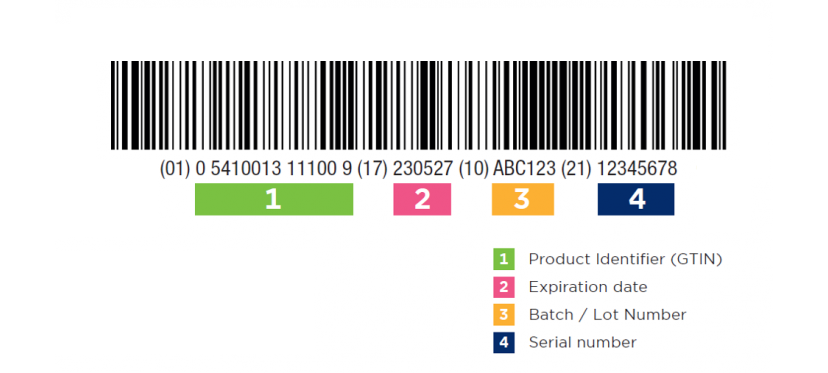
UDI Number
The Unique Device Identification (UDI) is a system used to mark and identify medical devices. Its purpose is to allow clear identification of specific devices on the market and facilitate their traceability.
The medical device regulation and the in-vitro device regulation require a UDI to be assigned to all medical devices, except for custom-made or investigational devices. The UDI is an addition to, not a substitute for, the existing labelling requirements for medical devices.
Pictures
Pictures are a great way to describe and identify a product visually. Don’t overthink this. Simply add pictures of your product to your technical file that show clearly what the product looks like.
See the example below of a road light.

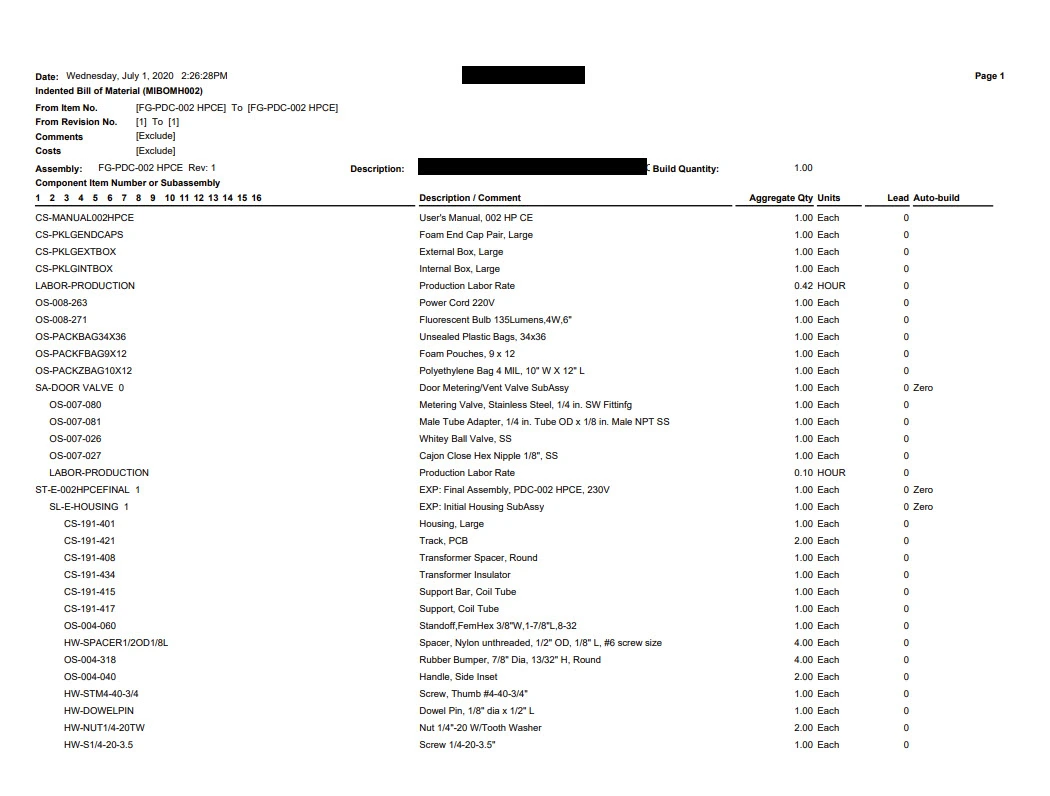
Bill of Materials (BOM)
A Bill of Materials (BOM) is a list of the raw materials, parts, sub-components and sub-assemblies and the quantities of each that the end products contain. The BOM helps explain to the market authorities how a product is structured or built up.
This is an example of what the bill of materials could look like:
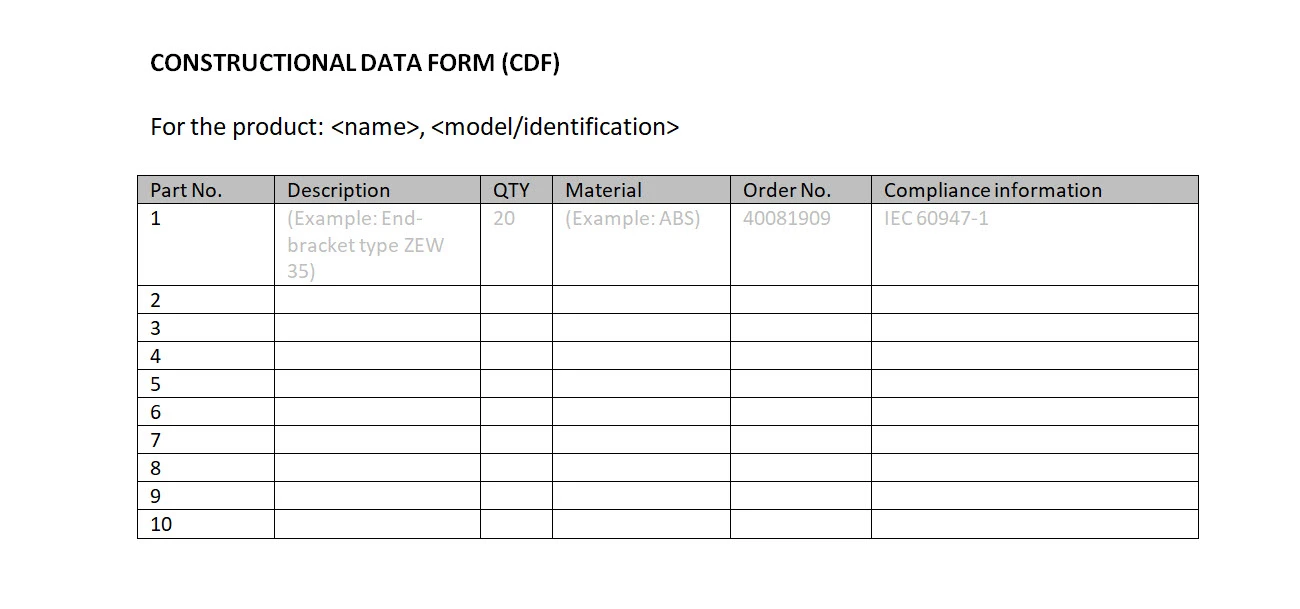
Constructional Data Form (CDF)
CDF stands for “Construction Data Form”. The CDF is a form showing compliance details for the (safety critical) components of a product. It is often used for machinery, personal protective equipment, electrical products and other products.
This is an example of what the constructional data form could look like:
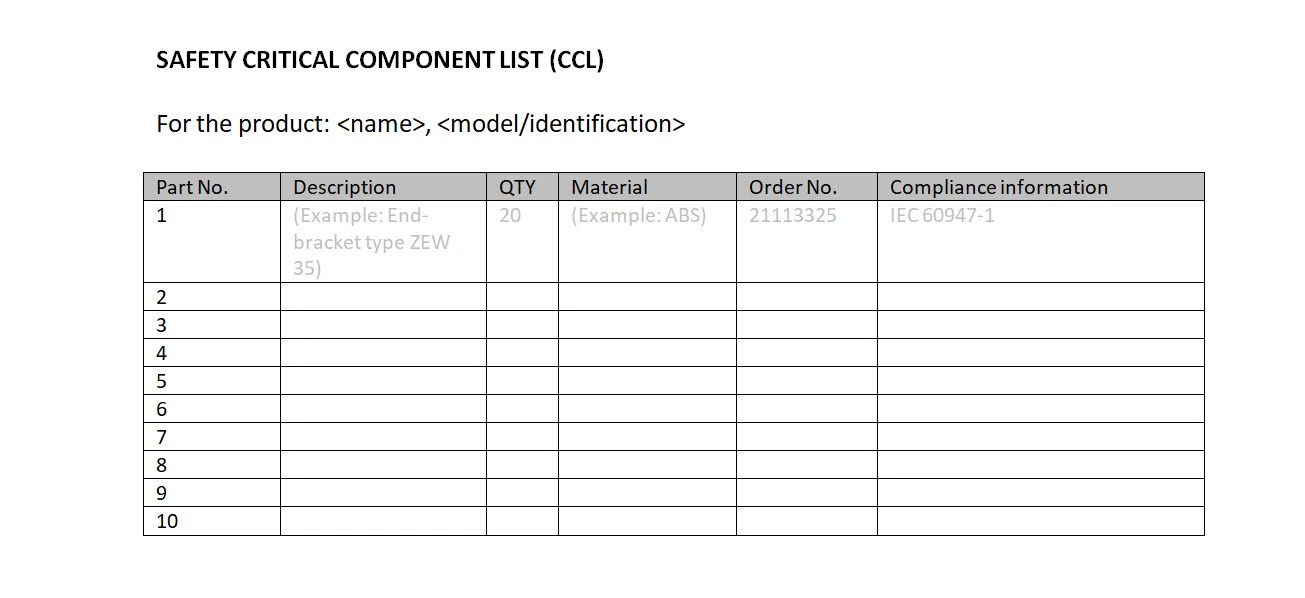
Safety Critical Components List (CCL)
CCL stands for "Critical Components List". The CCL lists all critical components used in a product. If critical components have a failure then the safety could be breached and changing one of the critical components may affect the safety of the product. By documenting the critical components, it is easier to trace if a certain event has been the result of critical component failure. CCL and CDF are often combined and included in test reports. If available, you may want to add the name of the technical data sheet in an additional column.
This is an example of what the safety critical component list could look like:
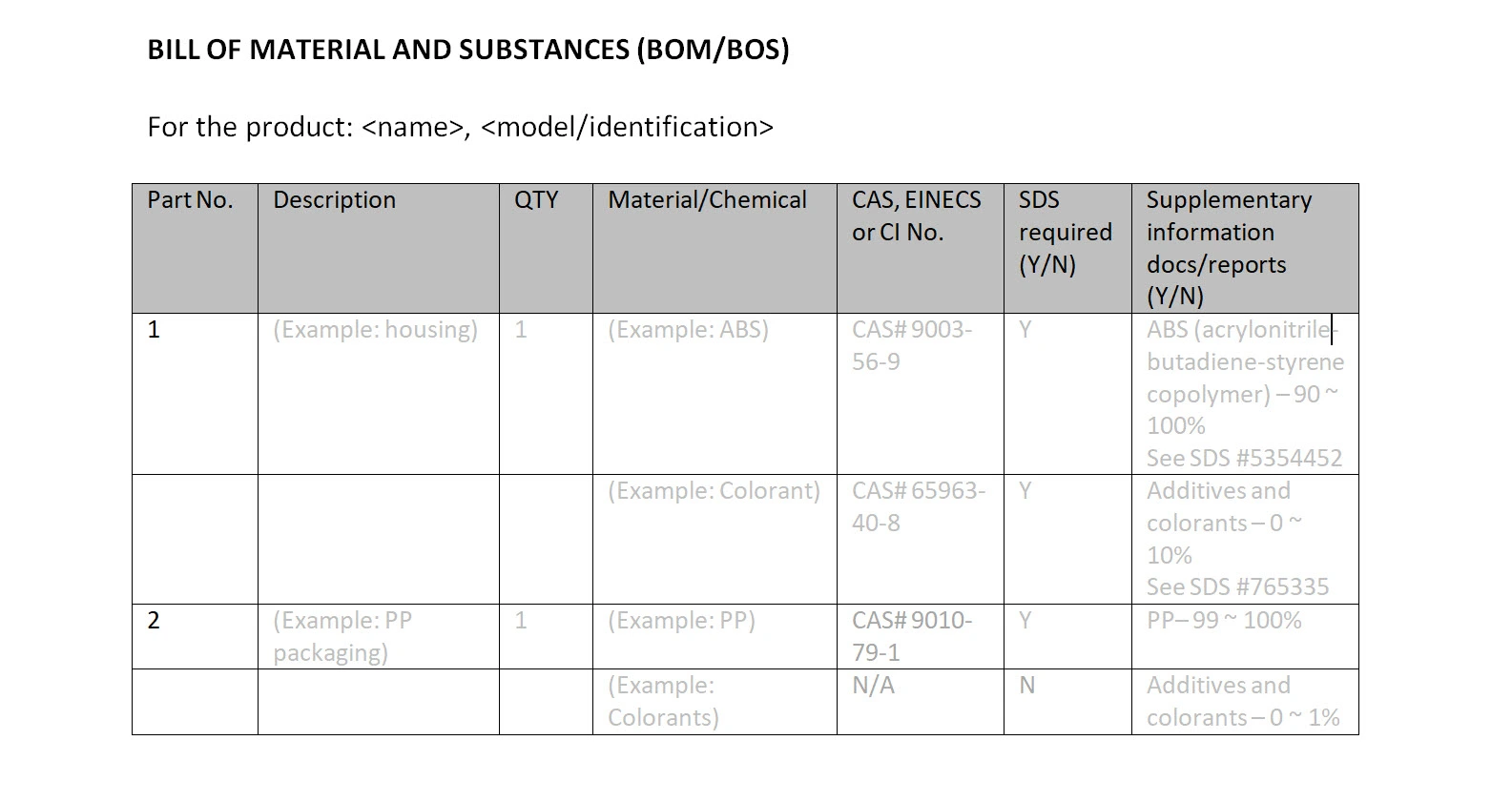
Bill of Substances (BOS)
A Bill of Substances (BOS) is relevant for the assessment of chemical, environmental and occupational hazards.Typically, products such as toys, food contact materials, and GPSD products may require a BOS.
Although there are many possible forms, this is an example of how the Bill of Substances (combined with the BOM) could look:
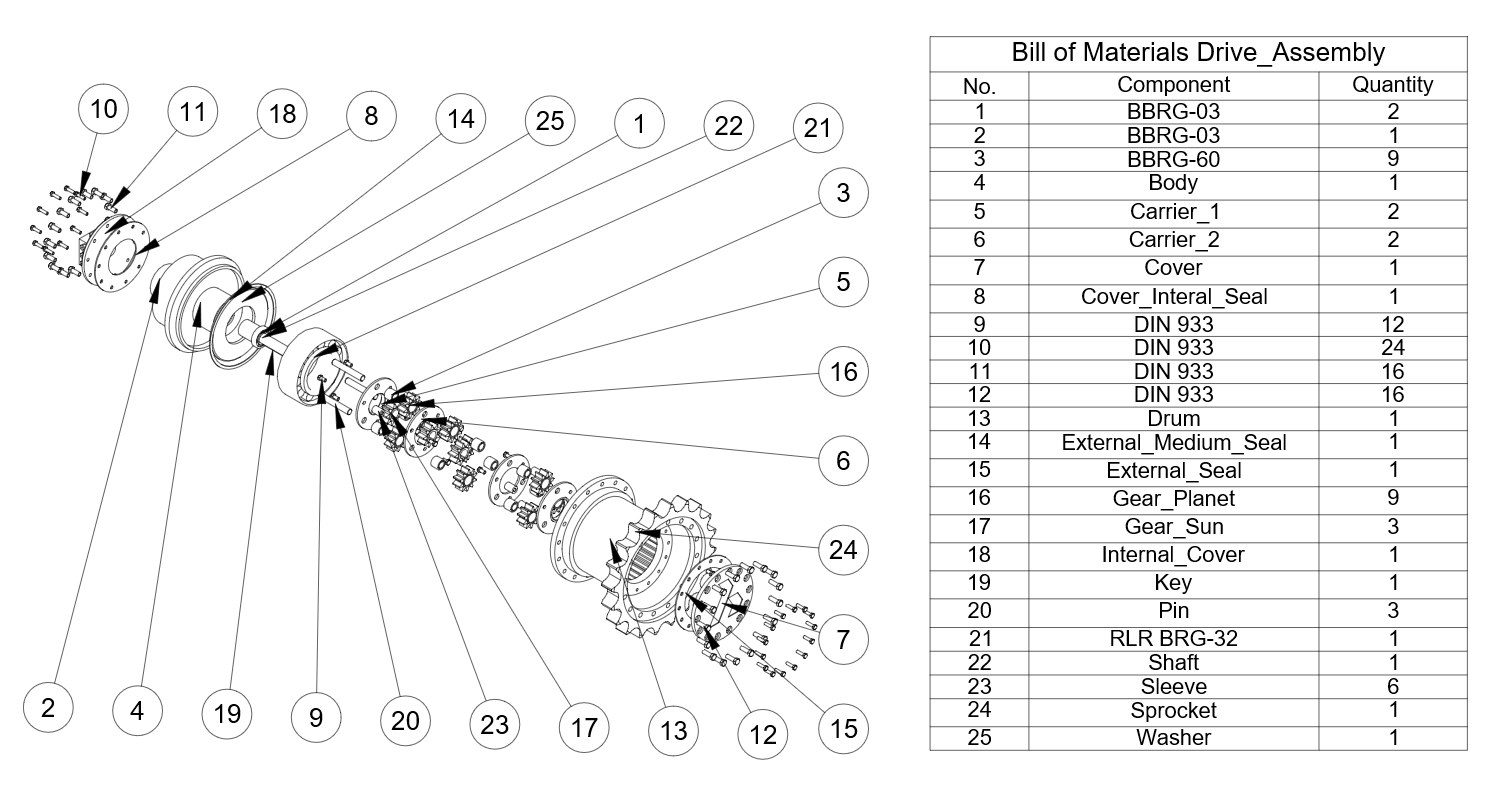
Mechanical drawings or exploded view
Mechanical drawings or an exploded view can be used to visualise the design of a product or a component. You can create detailed drawings or exploded views of any product, from multi-layer cables and their connectors to complex machinery.
As most products are designed using 3D CAD software, mechanical drawings and exploded views can easily be created using these tools. The drawings are typically used for manufacturing and assembly, but can also be used in your technical file to explain a product.
Although these kinds of drawings are rarely required by market surveillance authorities (as they are typically not crucial to determine safety critical aspects of a product), they are a great way to secure design knowledge within the company. So a technical file is not only intended for market authorities, but also serves internal purposes.
Exploded views or mechanical drawings can be combined with the bill of materials. They are also often used to indicate spare parts.
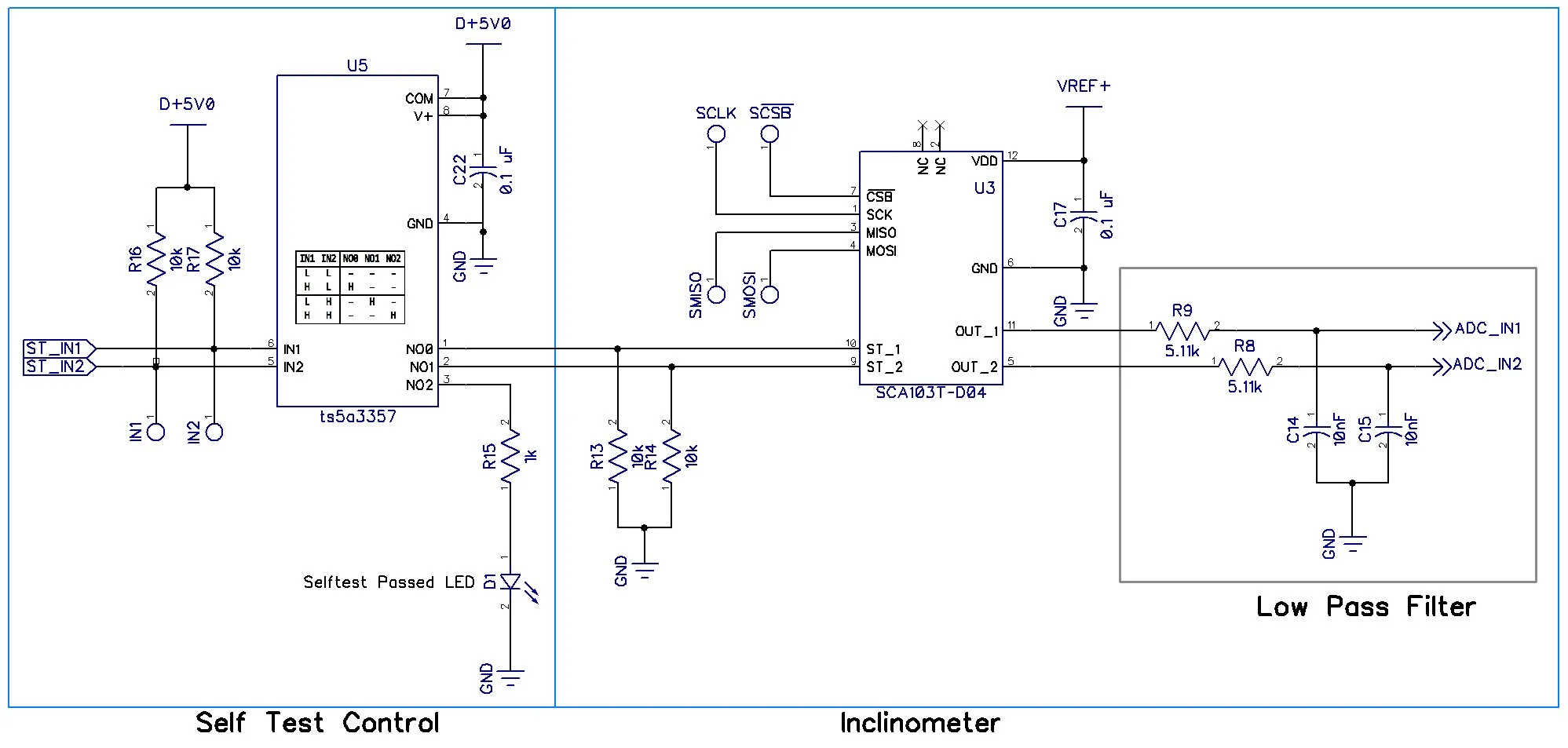
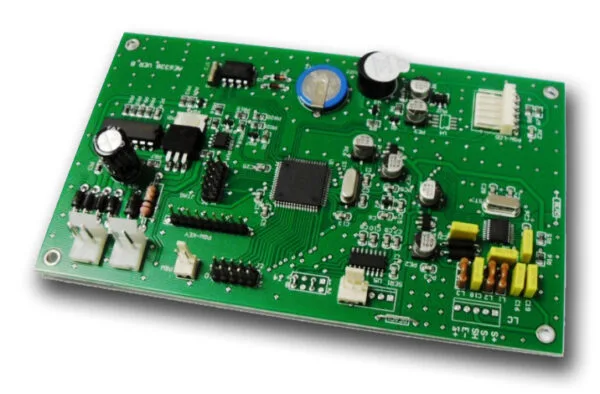
Printed circuit board (PCB) - diagrams and layouts
A printed circuit board (PCB) is used to connect electronic components to one another and they are used in nearly all electronic products. As the functioning and often the safety of the product depends on the design of the PCB, directives such as the machinery directive and low voltage directive require you to include drawings and pictures of the PCB in the technical file.
Product Contact Points
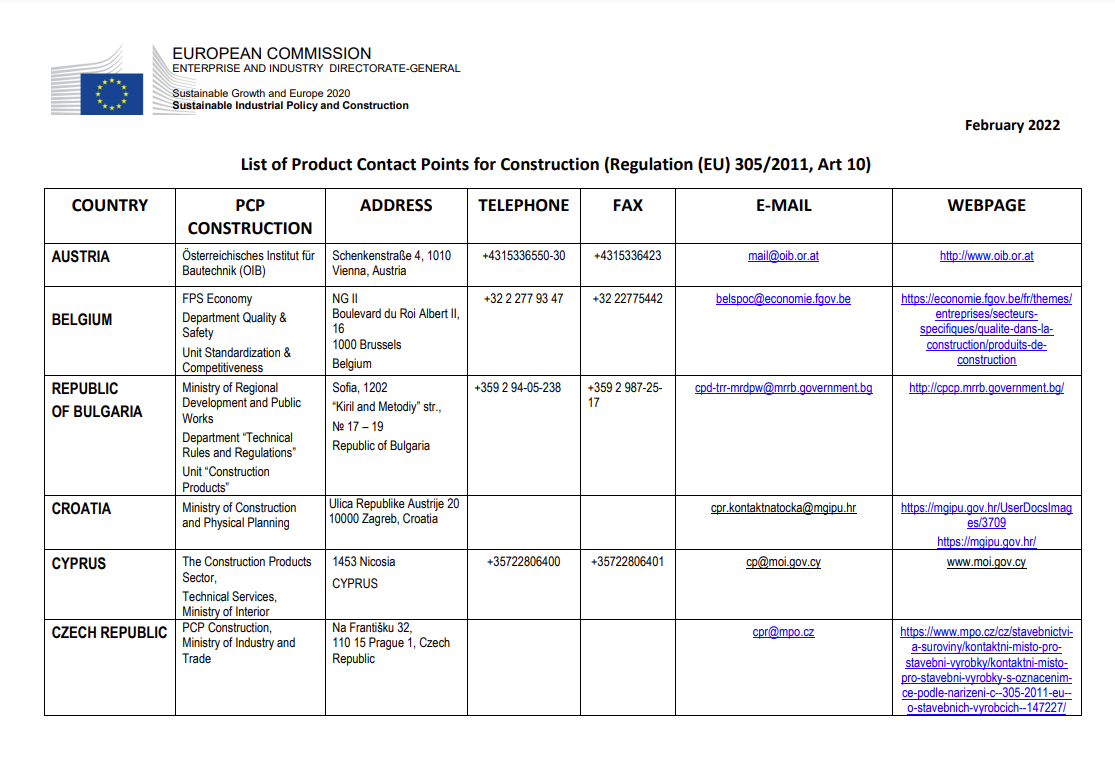
The construction products regulation (EU) 305/2011 in article 10 requires its member states to provide information on their rules and regulations for construction products through national contact points.
The technical file for construction products could contain an overview of the contact points in the countries where the product is sold, plus an overview of their guidelines in the application of requirements and the test methods given.
Safety documentation
Typically, safety critical components also come with a technical data sheet. Your technical file should have a folder containing all data sheets for the safety critical components or materials. This is a requirement for machinery and personal protective equipment, but applies basically to all products containing critical components.
Firmware, embedded software, source code & programmed logic
The machinery regulation requires the technical file to include, if applicable, the source code or programmed logic of the safety related software.

Sensor data characteristics, capabilities and limitations
The machinery regulation requires the technical file of sensor-fed, remotely-driven, or autonomous machinery to include, if the safety related operations are controlled by sensor data, a description of the general characteristics, capabilities and limitations of the system, data, development, testing and validation processes used.

EHSR checklists and applied standards
Requirements can be found in directives/regulations and in standards.
In some CE directives/regulations, such as the machinery directive/regulation and personal protective equipment regulation, these requirements are called the Essential Health and Safety Requirements (EHSR). The medical device regulation calls them the General Safety and Performance Requirements.
For machinery, a risk assessment shall be carried out in order to determine the health and safety requirements which apply to the machinery.
The technical file should contain an overview of the applicable requirements from both the directives/regulations and standards.
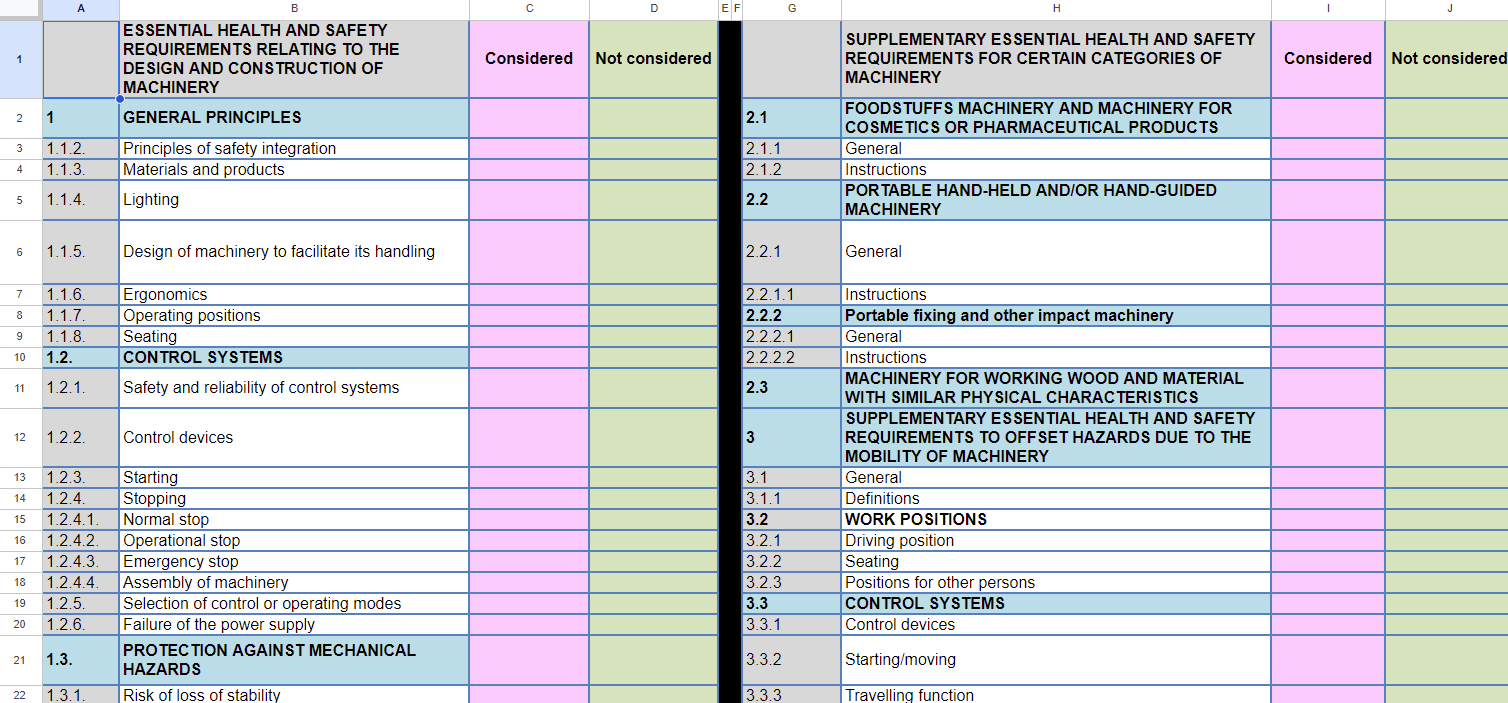
List of (EHS) Requirements
The technical file should contain an overview of the applicable requirements from the directives/regulations.
The example below contains an overview of essential health and safety requirements relating to the design and construction of machinery.
Applied standards and specifications
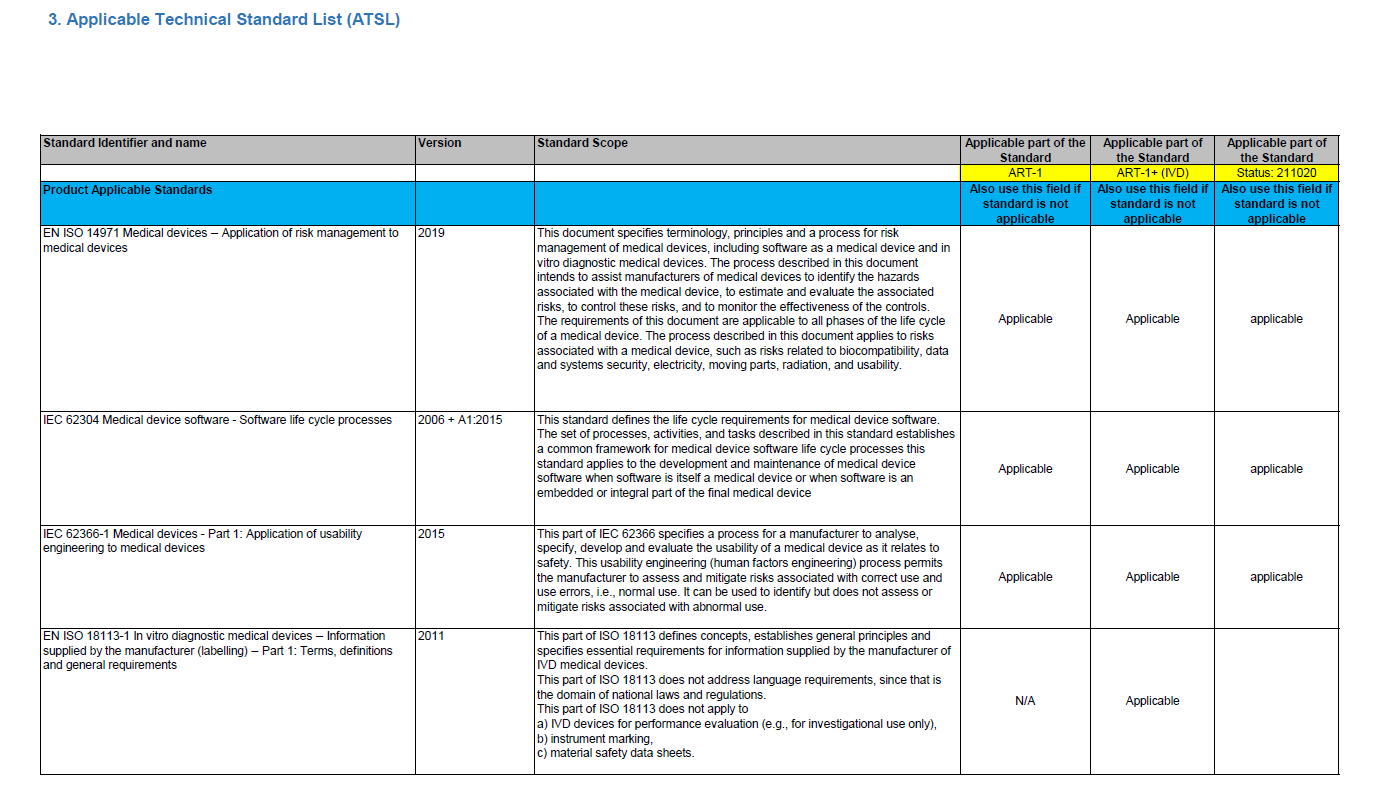
The technical file should contain an overview of the applicable requirements from applied standards and specifications.
The example below shows a section of the list of applied standards for an in-vitro medical device.
Design calculations, inspections and examinations
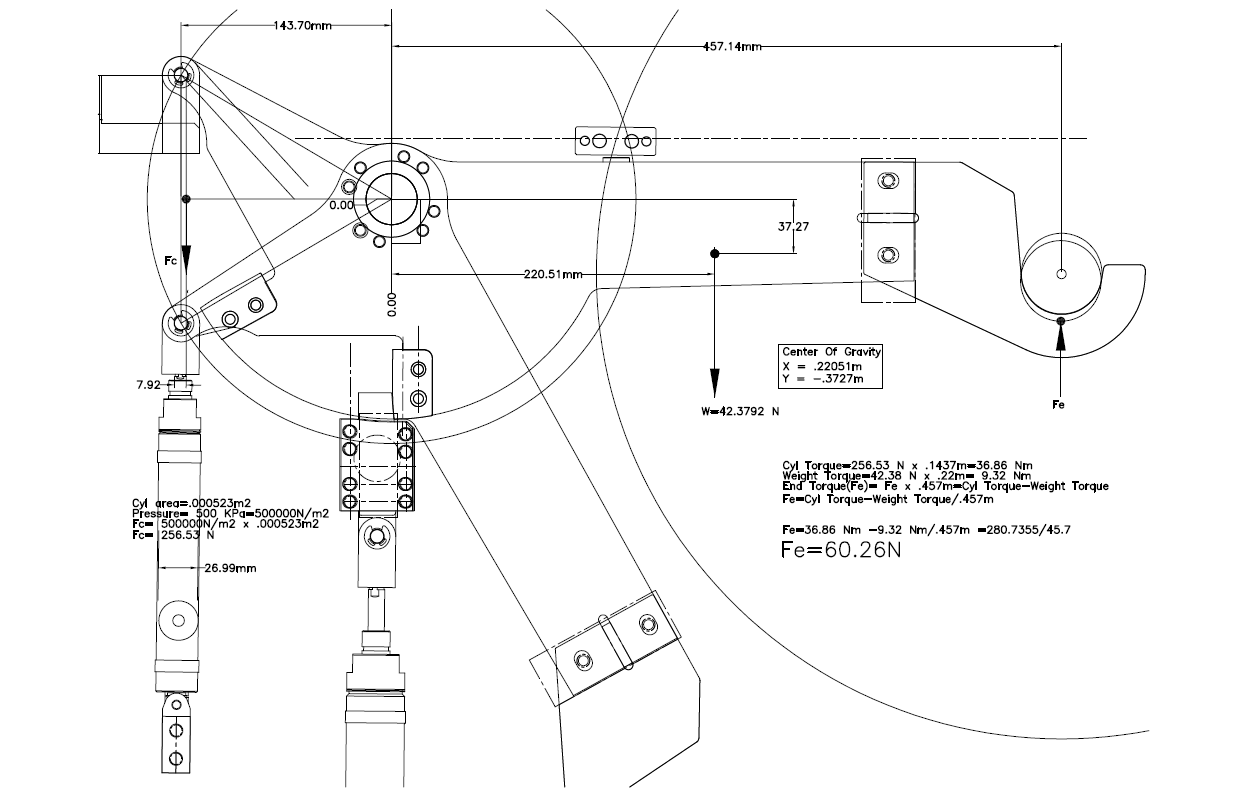
To verify the conformity of a product with the applicable (essential health and safety) requirements, the technical file shall include the results of the design calculations, inspections and examinations carried out.
For example, for machinery, the manufacturer must carry out necessary research and tests on components, fittings or the (partly) completed machinery to determine whether by its design or construction it is capable of being assembled, put into service or used safely.
The results and reports must be included in the technical file. Results of calculations, inspection and examinations can be of any kind. The example below shows the force calculation of a support arm.
Risk assessments
Risk assessment is a crucial element for all products placed on any market. A risk assessment consists of a series of logical steps to enable, in a systematic way, the analysis and evaluation of the risks associated with a product.
There are many ways to conduct a risk assessment. For machinery, ISO 12100 can be followed.
These templates help you creating your risk assessment:
Risk Assessment Template for Machinery
Risk Assessment Template for Other Products
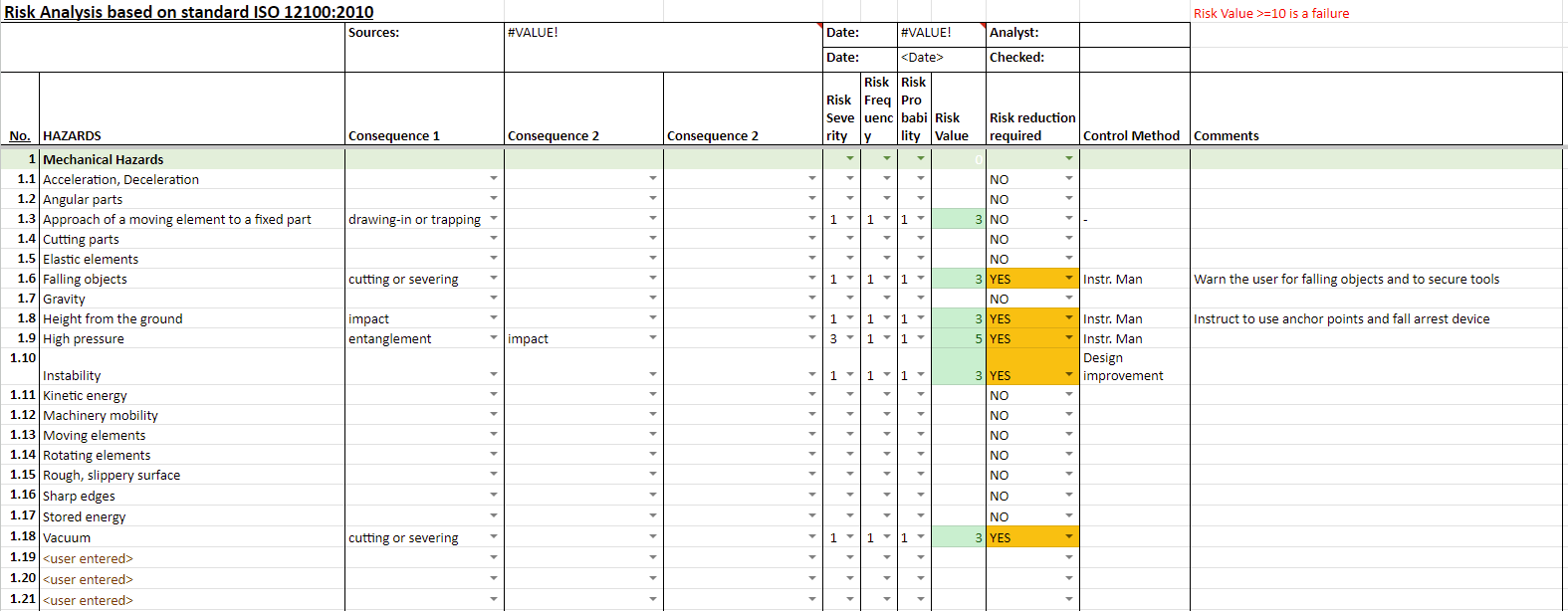
This is how a risk assessments could look like:
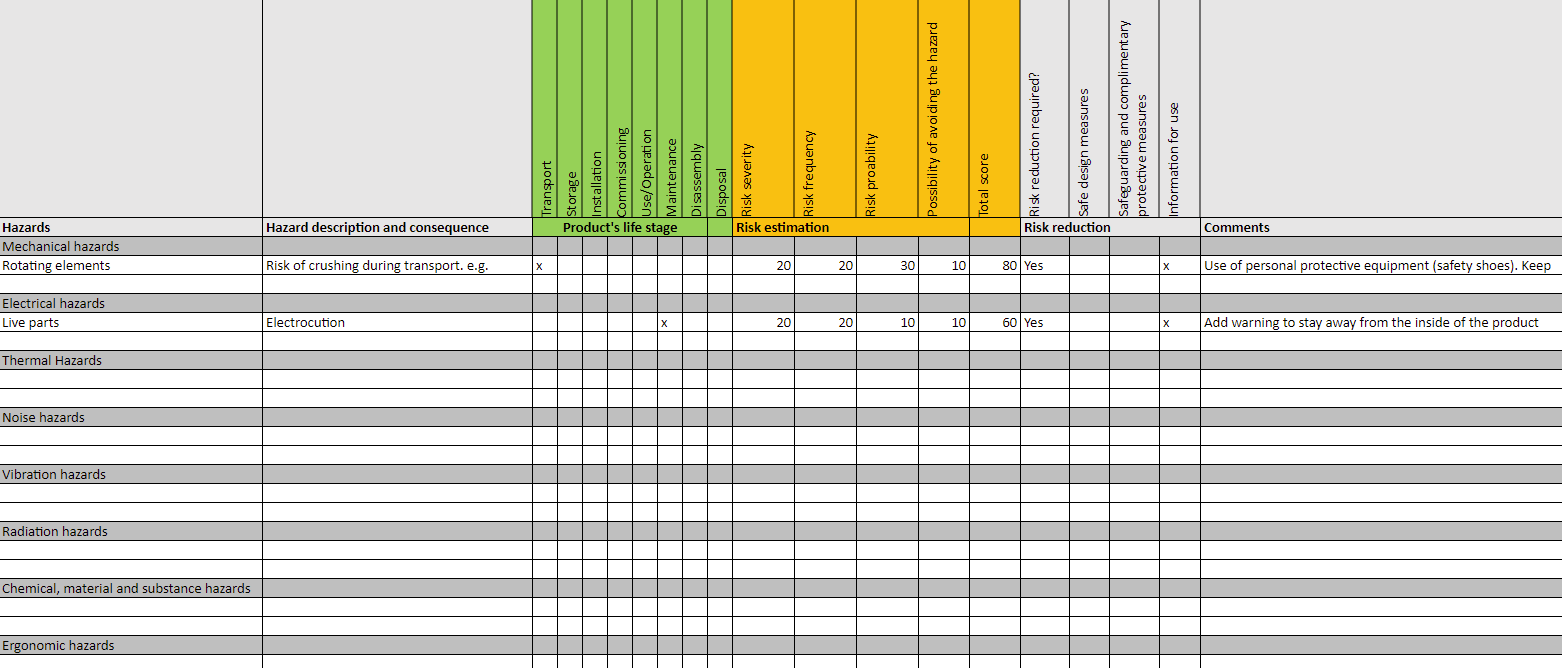
Here's an example of a risk assessments according to ISO 12100:
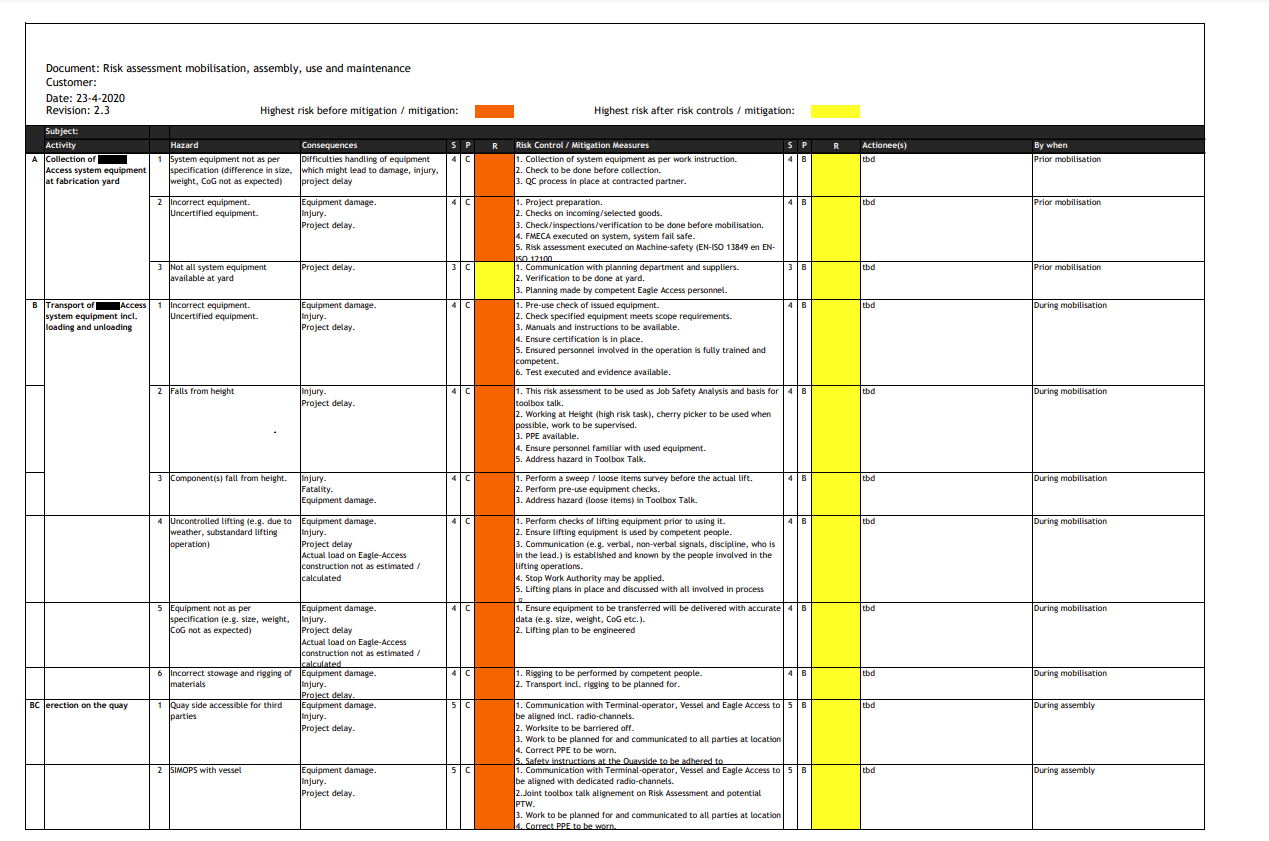
This is an example of a risk assessment created for offshore equipment:
User instructions, markings, labels, information
Basically every technical file must contain a copy of the user instructions, the markings on the product and packaging and any other relevant information for the user. All these things are sometimes also referred to as the external technical documentation: the documentation and information intended for the end-user of the product.
User manuals
Most directives require to provide user instructions with products. A copy should be stored in the technical file. That can be the user manual, installation instructions, maintenance manual, safety instruction or any other type of instructions.

Assembly instructions for partly completed machinery, if applicable
The Machinery Directive (2006/42/EC) uses the term partly completed machinery in the meaning of “an assembly of parts which is almost machinery but which cannot in itself perform a specific application. The partly completed machinery must be incorporated into or assembled with other machinery (or other partly completed machinery) to form a complete machinery that also comes under the scope of the Machinery Directive.”
For example, a robot can be considered a partly completed machine, as some robots need to be integrated into other machinery to carry out their functions.
The technical file for partly completed machinery shall contain the assembly instructions for integrating it with the machinery.
Markings, ratings, warnings & pictograms
For most products there are certain requirements for the product marking. Sometimes these are given in the directive, but more often you find these requirements in the relevant standards.
The technical file shall contain pictures of the product showing where these warnings are placed.


CE marking
Products falling under CE marking, must bear the CE marking. According to most directives, the CE marking shall be affixed visibly, legibly and indelibly on the product.
The technical file shall contain pictures of the product showing where the CE marking is placed.

Type ID plate
Machinery must be marked with several things, such as the CE marking, the business name of the manufacturer, designation of the machinery and the year of construction.Typically, this information is placed on the machine’s Type ID plate.
The technical file shall contain pictures of the machinery showing where theType ID plate is located.
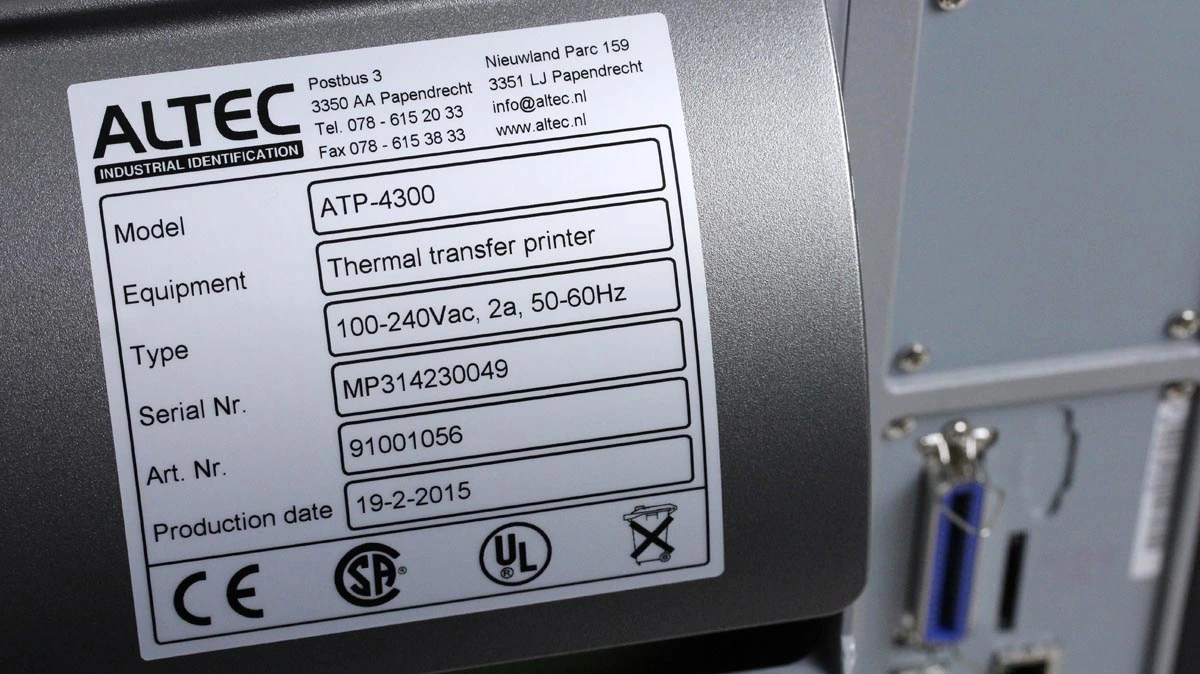
WEEE symbol
All electrical and electronic equipment must bear the waste electrical and electronic equipment (WEEE) marking. The technical file shall contain pictures of the product showing where the WEEE marking is located.

Packaging
Product safety legislation considers packaging as an integral part of a product. Most directives give requirements on the information that may be placed on the packaging, in case the size of the product does not allow placing certain mandatory markings on it.
In addition to that, there is the packaging directive, containing requirements on, for example, providing information on the packaging material used. Also REACH may apply to most packaging. For that reason, the technical file shall contain information on the packaging.
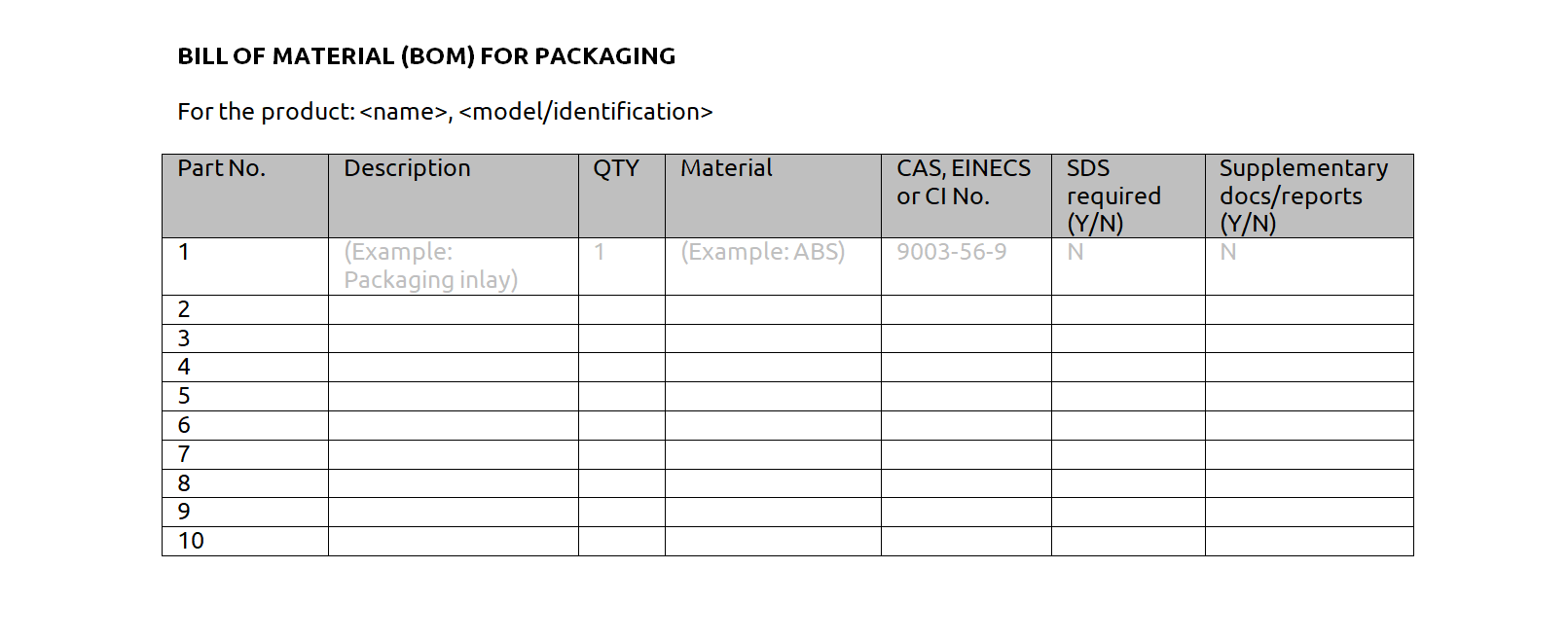
Bill of materials (BOM) of the packaging
Market surveillance authorities often check packaging components for REACH compliance. The packaging BOM provides an overview of all materials and chemicals used in the packaging.
Declaration of product identity of the packaging
If the packaging has been tested to comply with REACH, for example, the test results will be described in a test report.
When the packaging has been purchased outside the EU, the non-EU OEM manufacturer may have conducted the testing of the packaging. The test report will most likely mention the original packaging’s name, and not the name under which you market your product including its packaging. If this is the case, you need to declare that the packaging to which the test report applies, is the same packaging that you sell under your own name.
Here is a template that you can use to create your own declaration of product identity of the packaging:
Declaration of Product Identity of the packaging
We (supplier) hereby confirm that the following packaging as mentioned in the test report:
…………………………………….
is identical to the following packaging which is placed on the market:
…………………………………….
Confirmed by:
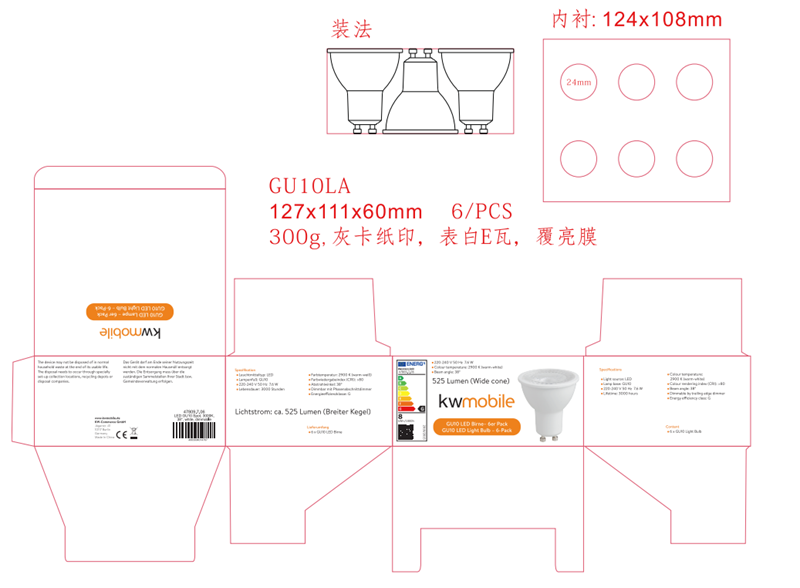
Picture, artwork of the packaging
Most directives contain requirements on the information that may be placed on the packaging, such as information on the packaging material or recycling information. Also the CE marking, an EU address, the model or type of the product, instructions and warnings are often placed on the packaging. For that reason, a copy of the packaging’s artwork shall be part of the technical file.
Test reports
Often, to prove compliance with certain requirements, a test may be conducted. You can test a toy for flammability using the standard EN 71-2, test the electromagnetic compatibility of an electrical device by using one of the many available EMC standards, or test a product for the presence of certain chemicals.
If you are not sure what to test for, you should do a requirements analysis first (step 1 & 2 of the CE marking process).
If your supplier has sent you test reports, but you’re unsure if these are valid and everything you need, you would also need to conduct a gap analysis.
Send us a message if you need help with this.
Safety of electrical equipment
Electrical equipment needs to be safe. What the definition of "safe" is varies from product to product.
There are many standards harmonised under the low voltage directive 2014/35/EU giving requirements for safety of electrical equipment, such as standards for audio and video equipment, lamps or switchgear.
You may be able to conduct a test on electrical safety yourself. If not, you can ask a third party to do the test for you. A test report can serve as your proof of compliance with some, most or even all of the requirements listed in Annex I of the low voltage directive.
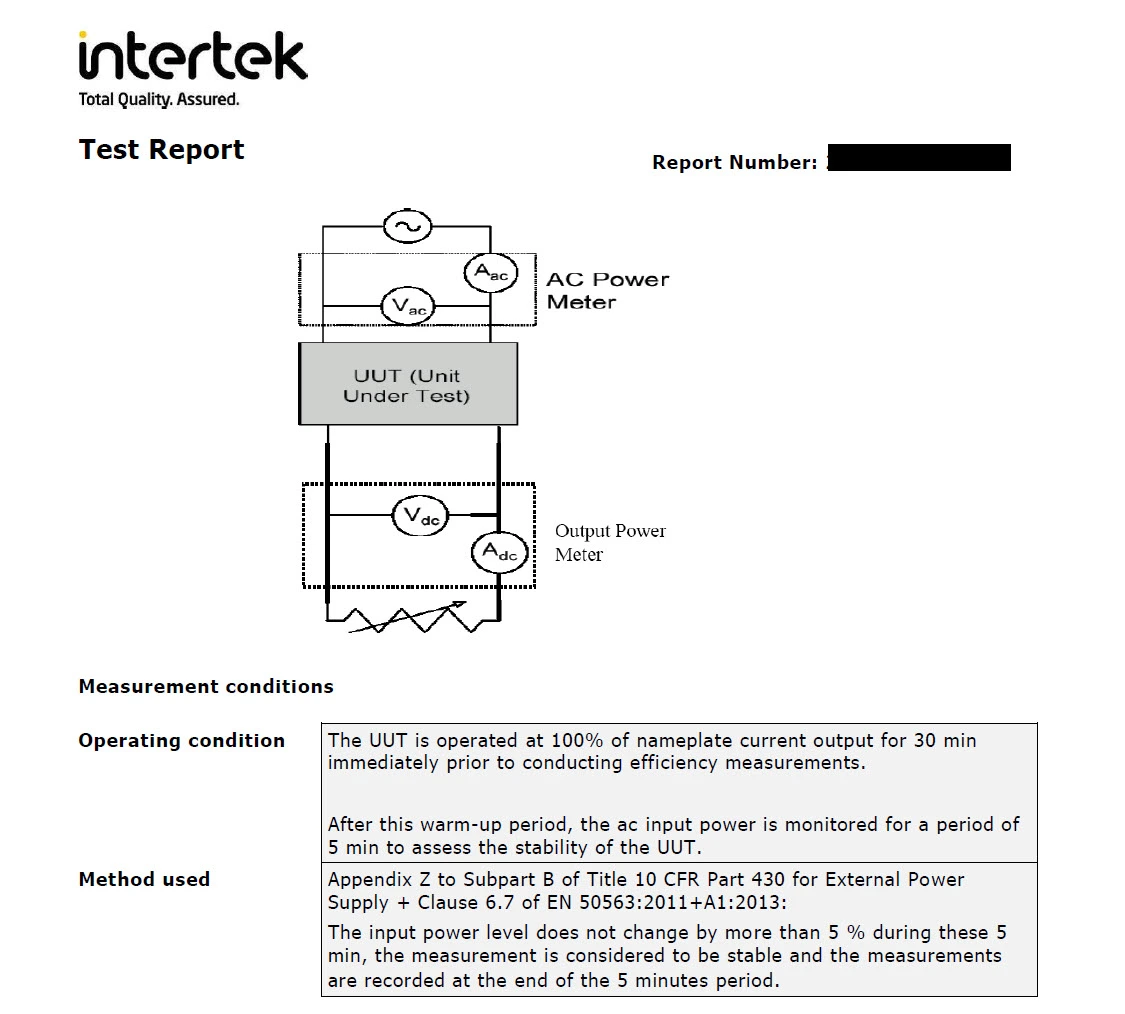
Screenshot taken from a test report on electrical safety for a fast charger
EMC - Test report
There are also many standards harmonised under the electromagnetic compatibility (EMC) directive 2014/30/EU. The EMC directive regulates the electromagnetic compatibility of electrical and electronic equipment.
You may be able to conduct an electromagnetic compatibility test yourself. If not, you can ask a third party to do the test for you. The test report will be your proof of compliance with the general requirements of Annex I of the EMC directive.


Electromagnetic fields (EMF) - Test report
EN 62311:2020 is a standard harmonised under the low voltage directive and provides requirements on the assessment of electronic and electrical equipment related to human exposure restrictions for electromagnetic fields.
The test report can serve as your proof of compliance with requirements 2b (radiation) of Annex I of the low voltage directive.


Radio equipment - Test report
Products emitting radio waves, such as products with Wi-Fi or Bluetooth, must comply with the radio equipment directive (RED). The RED contains requirements for the protection of health and safety of persons and of domestic animals and the protection of property.
There are many different standards for as many different types of equipment emitting radio waves. Conducting a test according to one of these standards proves compliance with the essential requirements of the RED.


Restriction of the use of certain hazardous substances (RoHS) - Test report
The RoHS directive 2011/65/EU applies to electrical and electronic equipment (EEE) and lays down rules on the restriction of the use of hazardous substances.
The use of the following substances is restricted:
- Lead (Pb)
- Mercury (Hg)
- Cadmium (Cd)
- Hexavalent chromium (Cr6+)
- Polybrominated biphenyls (PBB)
- Polybrominated diphenyl ether (PBDE)
- Bis(2-ethylhexyl) phthalate (DEHP)
- Butyl benzyl phthalate (BBP)
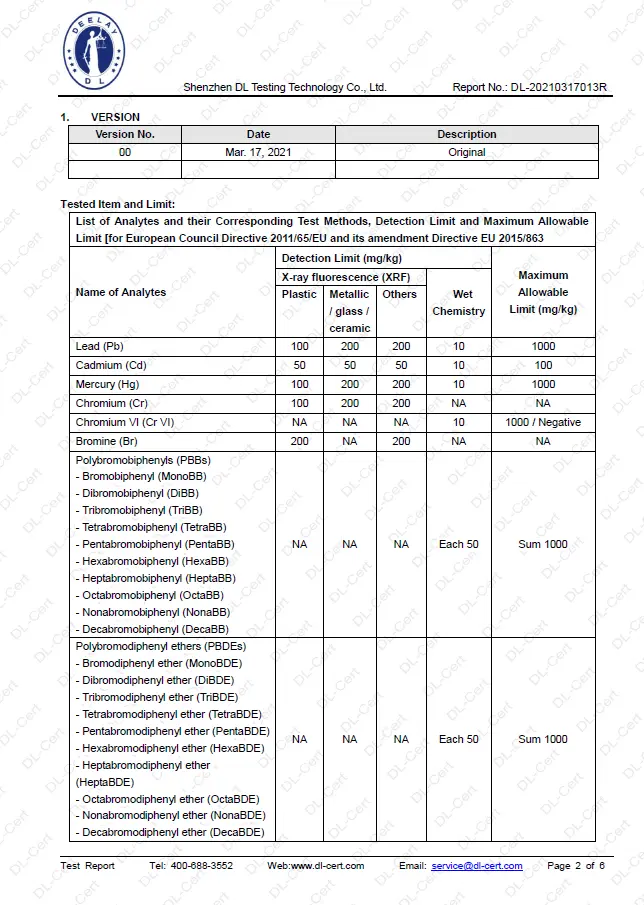
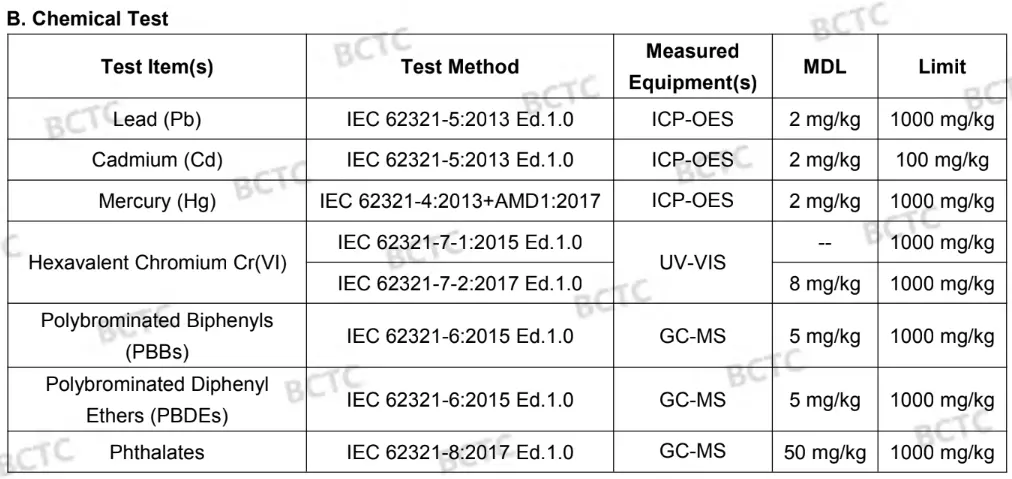
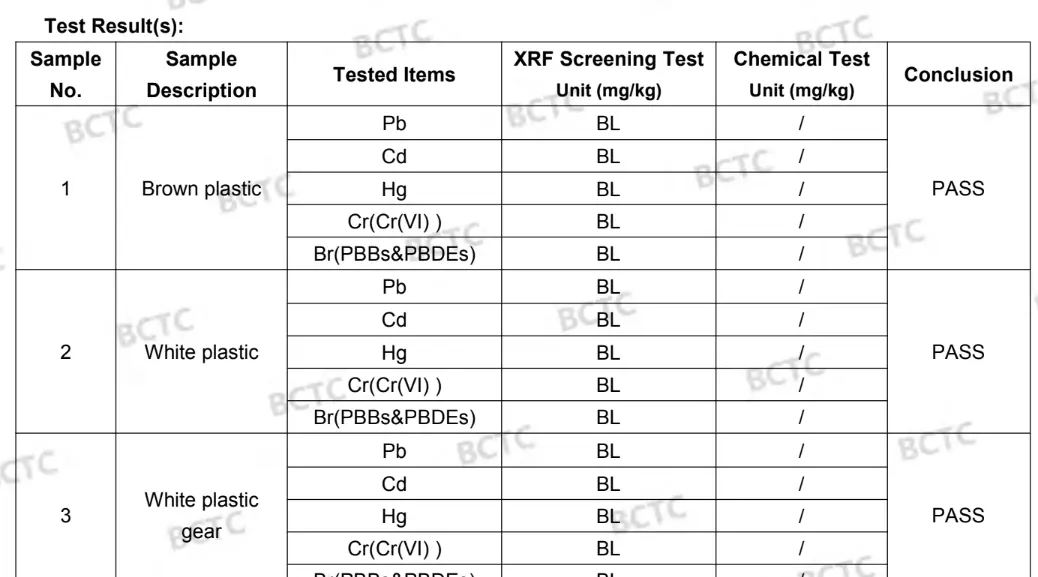
REACH - Test report
Although the REACH regulation is not a CE regulation, proof of compliance with REACH typically should be part of your technical file.
REACH stands for Registration, Evaluation, Authorisation of Chemicals and regulates the production, use and sale of chemicals.
REACH applies to substances (e.g. alcohol), mixtures (e.g. whiskey) and articles (the bottle). Although articles are considered as well, REACH is primarily about substances and mixtures as a lot of the REACH obligations are not applicable to articles.
An important aspect of REACH is a database, or list, of substances. This REACH list contains over 100,000 chemicals, of which 45 are banned and 211 are on the candidate list. Any new substances that are not on the REACH list are called (surprise) “new substances”.
New substances need to be approved before being put on the market
REACH requires anyone placing a substance on the EU market in quantities greater than one tonne per year to register that substance with the European Chemicals Agency (ECHA).
REACH can best be explained by means of a few examples:
- When a newspaper imports three tonnes of ink for producing their newspapers, they have to register the substance with ECHA.
- A book is an article. If you import one million books containing three tonnes of ink in total (which is more than the threshold of one tonne), there is no need to register this with ECHA as articles are excluded from this requirement.
- If a substance on the REACH candidate list constitutes more than a certain percentage in an article, then the user needs to be informed. For example, when a certain listed plasticiser is put in plastics (such as in a telephone cable) by a manufacturer from a non-EU country, there is a maximum on the allowed amount of that substance in the article. Annex 17 of the REACH regulation contains restrictions on the amount of a substance allowed in particular articles.
- A candle is considered to be a mixture and not an article as REACH says that articles that intentionally release substances are included in REACH. When lit, a candle may release toxic chemicals. For this reason, when importing over one tonne of candles, this should be registered with ECHA.
- Chromium VI is a very dangerous substance, but needed for modern engines. Chromium VI is a so-called restricted chemical, and the use of it for engines is authorised.
- The yellow pigment in temporary street marking is a lead chromate. This marking is hard to manufacture in Europe as the substance is banned, so it has to be imported as an article with a restricted amount of the substance in the article. There is a report requirement for the article once the marking is taken off the road again and becomes waste.
You can prove REACH compliance through testing of each part and material, or through a declaration.

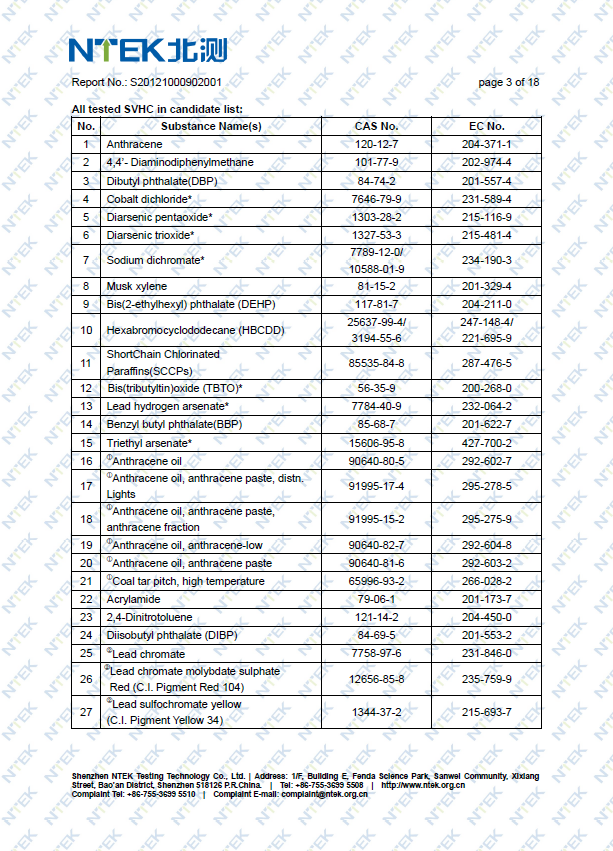
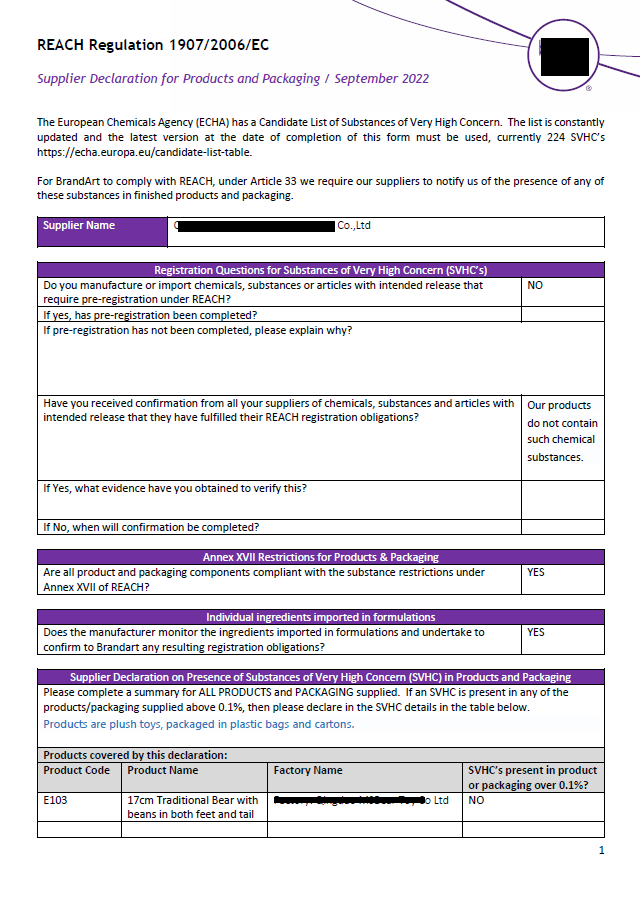

The following examples of test reports will be added soon:
- POP - Test report
- Energy related products and energy labeling - Test report
- Food contact materials - Test report
- Packaging materials - Test report
- Batteries - Test report
- Toy safety - Test reports (71-1, 71-2, 71-3)
- Construction products - Test report
- General product safety test report
- FCM Good Manufacturing Practice (GMP) audit report
- PPE test reports, protection class test reports
- Country specific requirements and test reports
Declarations
Drafting and signing the declaration of conformity (for most CE products), the declaration of incorporation (for partly completed machinery), or the declaration of performance (for construction products) is the last step in the CE marking process. A copy of the declarations shall be stored in the technical file.
Declaration of conformity
The declaration of conformity is a legal document that needs to be signed by the manufacturer. With the document the manufacturer formally declares the compliance of a particular product, which falls within the scope of CE marking, with the essential health and safety requirements of the relevant product safety directives.

Declaration of Incorporation for partly completed machinery, if applicable
The declaration of incorporation comes with incomplete machinery and basically states that the machinery is incomplete and only complies with some parts of the directive.

Declaration of performance
Construction products need to be provided with a declaration of performance (DoP). The DoP describes the construction product's characteristics, such as the extent to which it is watertight, resistant to wind, or thermal resistant.

The following examples of declarations will be added soon:
- Declaration of compliance for food contact materials
- Packaging materials - Declaration
Quality assurance
Quality assurance system - audit report or certificate
Manufacturers of machinery listed in Annex IV of the machinery directive must operate an approved quality system for design, manufacture, final inspection and testing of products.
The following examples of quality assurance will be added soon:
- Quality management system for medical devices
- Final Quality Control (FQC) - Documentation
- Incoming materials Quality Control (IQC) - Documentation
- Conformity assessment procedure documentation
Registration
The following examples of product registration will be added soon:
- SCIP notification report
- Third party certificates or licences
- Packaging
- Registration record WEEE
- Registration record EPREL
- Registration record UDI
- Registration record batteries
Do you have any technical documentation that could serve as a good example and help others? I would like to encourage you to submit material for this article in order to create an article that is as complete as possible.
 |
Ferry Vermeulen is a technical communication and compliance expert. He also is a parttime trainer at the Dutch standardisation institute (NEN). Listen to the INSTRKTIV podcast on Spotify or read one of his latest blog articles. Linkedin I Spotify I YouTube I Facebook I Twitter |
